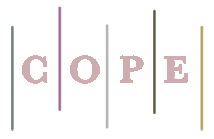Speciation of selenium (IV, VI) in urine and serum of thyroid patients by ultrasound-assisted dispersive liquid-liquid microextraction
Volume 3, Issue 03, Pages 5-17, Sep 2020 *** Field: Human Bioanalysis
Abstract
A simple in-vitro speciation of inorganic selenium (SeIV and SeVI) in serum blood and urine of hyperthyroidism and hypothyroidism patients based on isopropyl 2-[(isopropoxycarbothiolyl) disulfanyl] ethane thioate (IICDET) as a complexing agent were studied by ultrasound-assisted dispersive liquid-liquid bio-microextraction procedure (USA-DLLMBE). In first stage, 100 μL (≈0.1 g) of hydrophobic ionic liquid of [C8MIM][PF6] mixed with IICDET ligand and 100 μL of acetone. Then, the mixture injected to 10 mL of samples at pH=4. After shacking, the Se (IV) ions were complexed by IICDET and extracted to IL at pH=4 (R-S: …Se). The IL phase was separated from sample by centrifuging and inorganic selenium (Se IV) in remained samples was determined by electro thermal atomic absorption spectrometry (ET-AAS) after back extraction of Se (IV). As speciation, the Se (VI) reduced to Se (IV) in acidic pH (HCl, 130OC) and the total Se(T-Se) was obtained at pH=4.
References
G. Bjørklund, J. Aaseth, A.V. Skalny, J. Suliburska, M.G. Skalnaya, A.A. Nikonorov, Interactions of iron with manganese, zinc, chromium, and selenium as related to prophylaxis and treatment of iron deficiency, J. Trace Elem. Med. Biol., 41(2017) 41–53.
M. Kucharzewski, J. Braziewicz, U. Majewska, S. Góźdź, Concentration of selenium in the whole blood and the thyroid tissue of patients with various thyroid diseases, Biol. Trace Elem. Res., 88 (2002) 25–30.
M.K. Gürgöze, A. Olçücü, A.D. Aygün, E. Taskin, M. Kiliç, Serum and hair levels of zinc, selenium, iron, and copper in children with iron-deficiency anemia, Biol. Trace Elem. Res., 111 (2006) 23-29.
W. Chin-Thin, C. Wei-Tun, P. Tzu-Ming, W. Ren-Tse, Blood concentrations of selenium, zinc, iron, copper and calcium in patients with hepatocellular carcinoma, Clin. Chem. Lab. Med., 40 (2002) 1118-1122.
A.K. Baltaci, R. Mogulkoc, M. Belviranli, Serum levels of calcium, selenium, magnesium, phosphorus, chromium, copper and iron--their relation to zinc in rats with induced hypothyroidism, Acta Clin. Croat., 52 (2013) 151- 156.
G.F. Combs, Biomarkers of selenium status, Nutrients., 7 (2015) 2209–36
P.P. Felix, E.L. Ander, Urine selenium concentration is a useful biomarker for assessing population level selenium status, Environ. Int., 134 (2020) 105218.
D.L. Hatfield, P.A. Tsuji, B.A. Carlson, V.N. Gladyshev, Selenium and selenocysteine: Roles in cancer, health, and development, Trends Biochem. Sci., 39 (2014) 112–20.
W. Yang, A.M. Diamond, Selenium-binding protein as a tumor suppressor and a prognostic indicator of clinical outcome, Biomark Res., 1 (2013) 15.
Y. Wang, W. Fang, Y. Huang, F. Hu, Q. Ying, W. Yang, B. Xiong, Reduction of selenium-binding protein 1 sensitizes cancer cells to selenite via elevating extracellular glutathione: a novel mechanism of cancer-specific cytotoxicity of selenite, Free Radic. Biol. Med., 79 (2015) 186-196.
S. Misra, R.W. Kwong, S. Niyogi, Transport of selenium across the plasma membrane of primary hepatocytes and enterocytes of rainbow trout, J. Exp. Biol., 215 (2012) 1491–501.
Z. Huang, A.H. Rose, P.R. Hoffmann, The role of selenium in inflammation and immunity: from molecular mechanisms to therapeutic opportunities, Antioxid Redox Sign. 16 (2012) 705-743
N. Manevska, S. Stojanoski, T. Makazlieva, Selenium treatment effect in auto-immune hashimoto thyroiditis in macedonian population, J. Endocrinol. Metabol., 9 (2019) 22-28.
M. Leo, L. Bartalena., G. Rotondo Dottore, Effects of selenium on short-term control of hyperthyroidism due to Graves’ disease treated wif methimazole: results of a randomized clinical trial, J.Endocrinol. Invest., 40 (2017) 281–287.
G. Ira Martin, R. James, Sowers thyroid and the heart, Am. J. Med., 127 (2014) 691–698.
M. Outzen, A. Tjonneland, E.H. Larsen, S. Friis, S.B. Larsen, J. Christensen, Selenium status and risk of prostate cancer in a Danish population, Brit. J. Nutr., 115 (2016) 1669‐77.
A. Dalia, T. Loh, A. Sazili, M. Jahromi, A. Samsudin, The effect of dietary bacterial organic selenium on growth performance, antioxidant capacity, and selenoproteins gene expression in broiler chickens, BMC Vet. Res., 13 (2017). http://doi:10.1186/s12917-017-1159-4.
S.S. Najim, Determination of some trace elements in breast cancer serum by atomic absorption spectroscopy, Int. J. Chem., 9 (2017) 1-6.
M. Krawczyk-Coda, Determination of selenium in food samples by high-resolution continuum source atomic absorption spectrometry after preconcentration on halloysite nanotubes using ultrasound-assisted dispersive micro solid-phase extraction, Food Anal Meth., 12 (2019) 128-135.
A. Terol, F. Ardini, A. Basso, M. Grotti, Determination of selenium urinary metabolites by high temperature liquid chromatography-inductively coupled plasma mass spectrometry, J. Chromatogr., 1380 (2015) 112–119.
K. Pyrzynska, A. Sentkowska, Liquid chromatographic analysis of selenium species in plant materials, TrAC, Trend Anal. Chem., 111 (2019) 128–138.
C.K Su, W.C. Chen, 3D-printed, TiO2 NP-incorporated minicolumn coupled with ICP-MS for speciation of inorganic arsenic and selenium in high-salt-content samples, Microchim. Acta., 185 (2018) 1–8.
M. Pettine, T.J. McDonald, M. Sohn, G.A.K. Anquandah, R. Zboril, V.K.A Sharma, Critical review of selenium analysis in natural water samples, Trends Environ. Anal., 5 (2015) 1–7.
T. Hu, L.P. Liu, S.Z. Chen, W.L. Wu, G.G. Xiang, Y.B. Guo, Determination of selenium species in cordyceps militaris by high-performance liquid chromatography coupled to hydride generation atomic fluorescence spectrometry, Anal. Lett., 51 (2018) 2316-2330.
A.S.A. Ibrahim, R. Al-Farawati, U. Hawas,Y, Shaban, Recent microextraction techniques for determination and chemical speciation of selenium, Open Chem., 15 (2017) 103–122.
L. Nyaba, J.M. Matong, K.M. Dimpe, P.N. Nomngongo, Speciation of inorganic selenium in environmental samples after suspended dispersive solid phase microextraction combined with inductively coupled plasma spectrometric determination, Talanta., 159 (2016) 174–180.
A.H. Panhwar; M. Tuzen, T.G. Kazi, Ultrasonic assisted dispersive liquid-liquid microextraction method based on deep eutectic solvent for speciation, preconcentration and determination of selenium species (IV) and (VI) in water and food samples, Talanta., 175 (2017) 352–358.
N. Motakef Kazemi, A novel sorbent based on metal–organic framework for mercury separation from human serum samples by ultrasound assisted- ionic liquid-solid phase microextraction, Anal. Methods Environ. Chem. J., 2 (2019) 67-78.
Copyright (c) 2020 Analytical Methods in Environmental Chemistry Journal

This work is licensed under a Creative Commons Attribution 4.0 International License.
JOURNAL PUBLISHING AGREEMENT
PLEASE PROVIDE US THE FOLLOWING INFORMATION,
Article entitled:
Corresponding author:
To be published in the journal:
Your Status
I am the sole author of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
I am one author signing on behalf of all co-authors of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
Please tick the above blanks (as appropriate), review the Journal Publishing Agreement, and then sign and date the document in black ink.
Published Journal Article: the author may share a link to the formal publication through the relevant DOI. Additionally theses and dissertations which contain embedded Published Journal Articles as part of the formal submission may be hosted publicly by the awarding institution with a link to the formal publication through the relevant DOI. Any other sharing of Published Journal Articles is by agreement with the publisher only.
Signed: ______________________________________ Name printed: ___________________________________________
Title and Company (if employer representative): _______________________Date: __________________________________