Ionic liquid functionlized on multiwall carbon nanotubes for nickel and lead determination in human serum and urine samples by micro solid-phase extraction
Volume 4, Issue 02, Pages 72-85, Jun 2021 *** Field: Analytical Biochemistry
Abstract
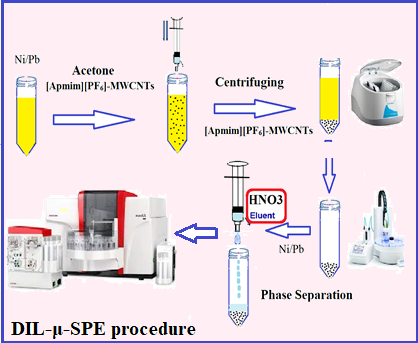
In this study, a novel synthesis adsorbent, 1-(3-aminopropyl)-3-methylimidazolium hexafluorophosphate functionlized on multiwall carbon nanotubes ([Apmim][PF6]-MWCNTs, IL@MWCNTS) was used for nickel/lead (Ni/Pb) extraction and determination by dispersive ionic liquid micro solid-phase extraction (DIL-μ-SPE) coupled to electrothermal atomic absorption spectrometry (ET-AAS). After dilution of 20 mg of IL@MWCNTS in 200 μL of acetone, the mixture was injected to 10 mL of human serum/urine samples at pH of 8.0. After sonication for 5 min, the Ni(II) / Pb(II) were extracted by ionic liquid phase and then centrifuged for 2.5 min. The upper liquid phase set aside and Ni(II) / Pb(II) loaded in adsorbent were back-extracted by acidic solution at pH=2-3. Finally, the concentration of total nickel and lead was determined by ET-AAS. By optimizing, the limit of detection, linear range, and enrichment factor for nickel and lead were obtained (0.05 μg L−1; 0.1 μg L−1), (0.2-5.8 μg L−1; 0.4-30 μg L−1) and 24.7; 5.1, respectively (RSD less than 5%). Also, the capacity absorption of IL@MWCNTS for nickel and lead ions were achieved 149.3 mg g-1 and 162.5 mg g-1, respectively. The DIL-μ-SPE procedure was validated for nickel and lead extraction by spiking of real samples and ICP-MS analyzer.
References
World Health Organization (WHO), Preventing disease through healthy environments: exposure to lead: a major public health concern, 2019.
Environmental Protection Agency (USEPA) Basic Information About Lead in Drinking Water, 2014.
Agency for Toxic Substances and Disease Registry, Division of Toxicology and Human Health Sciences, 1600 Clifton Road NE, Mailstop S102-1, Atlanta, GA 30333, revision 2019.
Agency for Toxic Substances and Disease Registry (ATSDR), Toxicological profile for Lead,Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service, 2019.
Agency for Toxic Substances and Disease Registry (ATSDR), Toxicological profile for Nickel. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service, 2005.
A.A. Ab Latif Wani, J.A. Usmani, Lead toxicity: a review, Interdiscip. toxicol., 8 (2015) 55-64.
G. Flora, D. Gupta, A. Tiwari, Toxicity of lead: a review with recent updates, Interdiscip. toxicol., 5 (2012) 47-58.
T. Dignam, R. B. Kaufmann, L. LeStourgeon, M. Jean Brown, Control of lead sources in the United States, public health progress and current challenges to eliminating lead exposure, J. Public Health Manag. Pract., (2019) S13–S22. PMC6522252, https://doi.org/10.1097/PHH.0000000000000889.
M. Kirberger, J.J. Yang, Structural differences between Pb2+ and Ca2+binding sites in proteins: implications with respect to toxicity, J. Inorg. Biochem., 102 (2008) 1901–1909.
J.S. Magyar, T.-C. Weng, C.M. Stern, D.F. Dye, B.W. Rous, J.C. Payne, B.M. Bridgewater, A. Mijovilovich, G. Parkin, J.M. Zaleski, Reexamination of lead (II) coordination preferences in sulfur-rich sites: implications for a critical mechanism of lead poisoning, J. Am. Chem. Soc., 127 (2005) 9495-9505.
United States Food and Drug Administration (USFDA), Elemental impurities guidance for industry, Department of Health and Human Services, p. 41, 2017.
B.C. Schwarcz, L. Chilton, B. Shirley, S. Seifert, Childhood lead exposure associated with the use of kajal, an eye cosmetic from Afghanistan Albuquerque, New Mexico, Morb. Mortal Wkly. Rep., 62 (2013) 917-919.
K.L. Caldwell, P.Y. Cheng, J.M. Jarrett, Measurement challenges at low blood lead levels, Pediatrics., 140 (2017) e20170272. https://doi.org/10.1542/peds.2017-0272
D.C. Bellinger, Neurological and behavioral consequences of childhood lead exposure. PLOS Med., 5 (2008) e115. https://doi.org/10.1371/journal.pmed.0050115.pdf.
A. Abbas, A.M. Al-Amer, T. Laoui, M.J. Al-Marri, M.S. Nasser, M. Khraisheh, Heavy metal removal from aqueous solution by advanced carbon nanotubes: critical review of adsorption applications, Sep. Purifi. Technol., 157 (2016) 141-61.
S. Feng, X. Wang, G. Wei, P. Peng, Y. Yang, Z. Cao, Leachates of municipal solid waste incineration bottom ash from Macao: Heavy metal concentrations and genotoxicity, Chemosphere., 67 (2007) 1133-1137.
S.K. Seilkop, A.R. Oller, Respiratory cancer risks associated with low-level nickel exposure: An integrated assessment based on animal, epidemiological, and mechanistic data, Regul. Toxicol. Pharm., 37 (2003) 173–190.
Agency for Toxic Substances and Disease Registry, Division of Toxicology and Human Health Sciences, 1600 Clifton Road NE, Mailstop S102-1, Atlanta, GA 30333, revision 2019.
Agency for Toxic Substances and Disease Registry (ATSDR), Toxicological profile for Nickel. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service, 2005.
A. Fadhil Khudhair, M. Khudhair Hassan, H. F. Alesary , A. S. Abbas, Simple pre-concentration method for the determination of nickel(II) in urine samples using UV-VIS spectrophotometry and flame atomic absorption spectrometry techniques, Indones. J. Chem., 19 (2019) 638 – 649.
S. Orecchio, D. Amorello, Determination of trace elements in gluten-free food for celiac people by ICP-MS, Microchem. J., 116 ( 2014) 163-172.
M. Arjomandi, H. Shirkhanloo, A review: Analytical methods for heavy metals determination in environment and human samples, Anal. Methods Environ. Chem. J., 2 (2019) 97-126.
J. Shan Qun, W. Xiang Yu, S. Jin Lyu, Analysis of nickel distribution by synchrotron radiation X-ray fluorescence in nickel-induced early- and late-phase allergic contact dermatitis in Hartley guinea pigs, Chinese Med. J., 132 ( 2019 )1959-1964.
A. Baysal, S. Akman, A rapid solid sampling method for determination of nickel and copper along human hair by ETAAS, Microchem. J., 98 ( 2011) 291-296
M. Eftekhari, M. Gheibi, M. Akrami, F. Iranzad, Solid-phase extraction of ultra-trace levels of lead using tannic acid-coated graphene oxide as an efficient adsorbent followed by electrothermal atomic absorption spectrometry; response surface methodology–central composite design, New J. Chem., 42 (2018) 1159-1168.
M. Rajabi M. Abolhosseini, Magnetic dispersive micro-solid phase extraction merged with micro-sampling flame atomic absorption spectrometry using (Zn-Al LDH)-(PTh/DBSNa)-Fe3O4 nanosorbent for effective trace determination of nickel(II) and cadmium(II) in food samples, Microchem. J., 159 (2020) 105450.
S. Azimi, Z. Es’haghi, A magnetized nanoparticle based solid-phase extraction procedure followed by inductively coupled plasma atomic emission spectrometry to determine arsenic, lead and cadmium in water, milk, Indian rice and red tea, Bull. Environ. Contam. Toxicol., 98 (2017) 830-836.
W. Ding, X. Wang, T. Liu., Preconcentration/extraction of trace bisphenols in milks using a novel effervescent reaction-assisted dispersive solid-phase extraction based on magnetic nickel-based N-doped graphene tubes, Microchem. J., 150 (2019) 104109.
M. Shirani, F. Salari, S. Habibollahi, A. Akbari, Needle hub in-syringe solid phase extraction based a novel functionalized biopolyamide for simultaneous green separation/preconcentration and determination of cobalt, nickel, and chromium (III) in food and environmental samples with micro sampling flame atomic absorption spectrometry, Microchem. J., 152 (2020) 104340.
S. M. Sorouraddin, M. A. Farajzadeh H. Nasiri, Picoline based-homogeneous liquid–liquid microextraction of cobalt(II) and nickel(II) at trace levels from a high volume of an aqueous sample, Anal. Methods, 11 (2019) 1379-1386.
L. Khoshmaram, Air-assisted liquid–liquid microextraction combined with flame atomic absorption spectrometry for determination of trace Pb in biological and aqueous samples, Int. J. Environ.Anal. Chem., 101 (2021) 838-848.
H. Shirkhanloo, S. Davari Ahranjani, A lead analysis based on amine functionalized bimodal mesoporous silica nanoparticles in human biological samples by ultrasound assisted-ionic liquid trap-micro solid phase extraction, J. Pharm. Biomed. Aanl., 157 (2018) 1-9.
H. Shirkhanloo, Z. Karamzadeh, A novel biostructure sorbent based on CysSB/MetSB@MWCNTs for separation of nickel and cobalt in biological samples by ultrasound assisted-dispersive ionic liquid-suspension solid phase micro extraction, J. Pharm. Biomed. Anal., 172 (2019) 285-294.
N. Esmaeili, J. Rakhtshah, E. Kolvari, H. Shirkhanloo, Ultrasound assisted-dispersive-modification solid-phase extraction using task-specific ionic liquid immobilized on multiwall carbon nanotubes for speciation and determinationmercury in water samples, Microchem. J., 154 (2020) 104632.
Copyright (c) 2021 Analytical Methods in Environmental Chemistry Journal

This work is licensed under a Creative Commons Attribution 4.0 International License.
JOURNAL PUBLISHING AGREEMENT
PLEASE PROVIDE US THE FOLLOWING INFORMATION,
Article entitled:
Corresponding author:
To be published in the journal:
Your Status
I am the sole author of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
I am one author signing on behalf of all co-authors of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
Please tick the above blanks (as appropriate), review the Journal Publishing Agreement, and then sign and date the document in black ink.
Published Journal Article: the author may share a link to the formal publication through the relevant DOI. Additionally theses and dissertations which contain embedded Published Journal Articles as part of the formal submission may be hosted publicly by the awarding institution with a link to the formal publication through the relevant DOI. Any other sharing of Published Journal Articles is by agreement with the publisher only.
Signed: ______________________________________ Name printed: ___________________________________________
Title and Company (if employer representative): _______________________Date: __________________________________