Management and removal of nitrate contamination of water at the source using modified natural nano zeolite
Volume 5, Issue 01, Pages 36-48, Mar 2022 *** Field: Method by Nanochemistry
Abstract
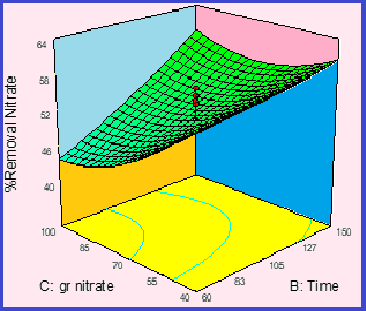
Nitrate is a hazardous substance for human health, the removal of which is an important environmental priority. Therefore, in this study, first, the sources of nitrate pollution of water were investigated, then the structure, role, and application of nanozeolites for the removal of nitrate ions were studied by the analytical method. Also, the presentation of management solutions, identification of polluting industrial sectors, different methods of removal and fabrication of ZSM-5/Fe/Ni nanosorbents, and the determination of optimal conditions for nitrate removal were investigated by experimental design software and graphical analysis of effective parameters. The results of graphical analysis of laboratory method showed us, the highest nitrate removal efficiency at a residence time of 150 minutes, pH 3, 4 g L-1 adsorbent, and 40 mg L-1 nitrate were achieved (%RE:91.5-97.4). Experimental results indicate the high efficiency, absorption capacity, and effectiveness of ZSM-5/Fe/Ni adsorbents for nitrate removal in waters. Finally, the absorbance values or nitrate concentrations between 20-120 mg L-1 were measured by the UV-Vis spectrophotometry. The maximum absorption capacity of ZSM-5/Fe/Ni adsorbents for nitrate was obtained 136.7 mg g-1. The developed method based on a novel ZSM-5/Fe/Ni adsorbents has many advantages such as simple, low cost, high efficiency, and favorite recovery.
References
T. Meftah, M. M. Zerafat, Nitrate removal from drinking water using organo-silane modified natural nano-zeolite, Int. J. Nanosci. Nanotechnol.,12 (2016) 223-232. http://www.ijnnonline.net/article_22931.html
B. Kamarehie, E. Aghaali, SA. Musavi, SY. Hashemi, A. Jafari, Nitrate removal from aqueous solutions using granular activated carbon modified with Iron nanoparticles, Int. J. Eng., Transactions A: Basics, 31 (2018) 554-563. https://doi.org/10.5829/ije.2018.31.04a.06
M. Mazarji, B. Aminzadeh, M. Baghdadi, A. Bhatnagar, Removal of nitrate from aqueous solution using modified granular activated carbon, J. Mol. Liq., 233 (2017) 139-148. https://doi.org/10.1016/j.molliq.2017.03.004
P. Revilla, M. Detras, V. Migo, C. Alfafara, Nitrate removal from aqueous solution by adsorption using municipal solid waste-derived activated biochar, IOP Conference Series: Mater. Sci. Eng., 778 (2020) 012135. https://doi.org/10.1088/1757-899X/778/1/012135.
H.A.T. Banu, P. Karthikeyan, S. Meenakshi, Removal of nitrate and phosphate ions from aqueous solution using zirconium encapsulated chitosan quaternized beads: Preparation, characterization and mechanistic performance, Results Surf. Interfaces, 3 (2021) 100010. https://doi.org/10.1016/j.rsurfi.2021.100010
L. Yang, M. Yang. P. Xu, X. Zhao, Characteristics of nitrate removal from aqueous solution by modified steel slag, Water, 9 (2017)757-774. https//doi/10.3390/w9100757
S. Chatterjee, D. Lee, S. Lee, M.S. Woo, Nitrate removal from aqueous solutions by cross-linked chitosan beads conditioned with sodium bisulfate, J. Hazard. Mater., 166 (2009) 508-513. https//doi/10.1016/j.jhazmat.2008.11.045
A. Bhatnagar, E. Kumar, M. Sillanpä, Nitrate removal from water by nano-alumina: characterization and sorption studies, Chem. Eng. J., 163 (2010) 317–323. https//doi/10.1016/j.cej.2010.08.008
G. Muradova, S. Gadjieva, L. Di Palma, Nitrates removal by bimetallic nanoparticles in water, Chem. Eng. Trans., 47 (2016) 205-210. https//doi/10.3303/CET1647035
L.E. Hanache, L. Sundermann, B. Lebeau, J. Toufaily, T. Hamieh, T.J. Daou, Surfactant-modified MFI-type nanozeolites: Super-adsorbents for nitrate removal from contaminated water, Micropor. Mesopor. Mater., 283 (2019) 1–13. https//doi/10.1016/j.micromeso.2019.03.049
A. Najdenkoska, Development of HPLC method for analysis of nitrite and nitrate in vegetable, J. Agricultural, Food Environ. Sci., 67 (2016), 33-39. https://core.ac.uk/download/pdf/287304048.pdf
V. Kmecl, T. Knap, D. Žnidarčič, Evaluation of the nitrate and nitrite content of vegetables commonly grown in Slovenia, Italian J. Agronomy, 12 (2017). 79-84. https://doi.org/10.4081/ija.2017.801
Association of official analytical chemists, guidelines for single laboratory validation of chemical methods for dietary supplements and botanicals, Association of official analytical chemists international, Maryland, 2002. https://pdf4pro.com/view/aoac-guidelines-for-single-laboratory-5b9252.html
M. Hassani, M. Zeeb, A. Monzavi, Z. Khodadadi, M. Kalaee, Adsorption of nitrate from aqueous solution with ZSM-5/Fe nanosorbent based on optimizing of the isotherms conditions before determination by UV-Vis Spectrophotometry, Anal. Methods Environ. Chem. J., 4 (2021) 49-63. https://doi.org/10.24200/amecj.v4.i04.154
M. Hassani, M. Zeeb, A. Monzavi, Z. Khodadadi, M. Kalaee, Response surface modeling and optimization of microbial fuel cells with surface-modified graphite anode electrode by ZSM-5 nanocatalyst functionalized, Chem. Methodol., 6 (2021) 253-268. https://doi.org/10.22034/CHEMM.2022.324312.1425
J. Rodríguez-Maroto, F. García-Herruzo, A. García-Rubio, C. Gómez-Lahoz, C. Vereda-Alonso, Kinetics of the chemical reduction of nitrate by zero-valent iron, Chemosphere, 74 (2009) 804-809. https//doi/10.1016/j.chemosphere.2008.10.020
S. Sepehri, M. Heidarpour, J. Abedi-Koupai, Nitrate removal from aqueous solution using natural zeolite-supported zero-valent iron nanoparticles, Soil Water Res., 9 (2014) 224–232. https://doi.org/10.17221/11/2014-SWR
B.W. Chieng, N. A. Ibrahim, Department of optimization of tensile strength of poly(Lactic Acid)/graphene nanocomposites using response surface methodology, Polymer-Plastics Technol. Eng., 51 (2012) 791–799. https//doi/10.1080/03602559.2012.663043.
Q. Zhang. G. Liu. L. Wang. X. Zhang, G. Li, Controllable decomposition of methanol for active fuel cooling technology, Energy Fuels, 28 (2014) 4431–4439. http://doi.org/10.1021/ef500668q.
Copyright (c) 2022 Analytical Methods in Environmental Chemistry Journal

This work is licensed under a Creative Commons Attribution 4.0 International License.
JOURNAL PUBLISHING AGREEMENT
PLEASE PROVIDE US THE FOLLOWING INFORMATION,
Article entitled:
Corresponding author:
To be published in the journal:
Your Status
I am the sole author of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
I am one author signing on behalf of all co-authors of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
Please tick the above blanks (as appropriate), review the Journal Publishing Agreement, and then sign and date the document in black ink.
Published Journal Article: the author may share a link to the formal publication through the relevant DOI. Additionally theses and dissertations which contain embedded Published Journal Articles as part of the formal submission may be hosted publicly by the awarding institution with a link to the formal publication through the relevant DOI. Any other sharing of Published Journal Articles is by agreement with the publisher only.
Signed: ______________________________________ Name printed: ___________________________________________
Title and Company (if employer representative): _______________________Date: __________________________________