Rapid analysis of chromium (III, VI) in water and wastewater samples based on Task-specific ionic liquid by the ultra-assisted dispersive ionic liquid-liquid microextraction
Volume 5, Issue 01, Pages 75-85, Mar 2022 *** Field: Analytical Chemistry
Abstract
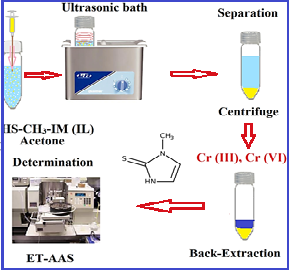
In this research, 2-mercapto-1-methylimidazole a novel Task-specific ionic liquid (C4H6N2S; HS-CH3-IM) was used with a new approach for speciation of Cr (III, VI) from water samples by ultra-assisted dispersive ionic liquid-liquid microextraction procedure (USA-D-ILLME). Due to the procedure, 100 mg of HS-CH3-IM and 0.2 mL of acetone were mixed and injected into 10 mL of water or standard Cr (III) and Cr (VI) solution in the conical tube. After stirring for 5 min, the Cr (VI) and Cr (III) were extracted with a positive and negative charge of the thiol group (HS2+, HS-) in pH 2 or 8 and pH 5, respectively. The Cr (III, VI) loaded on the HS-CH3-IM was back-extracted in a liquid solution. Finally, the concentration of the Cr (III, VI) ions in a remained solution were measured with ET-AAS . The total chromium was determined in water samples by summarizing the Cr (VI) and Cr (III) contents. All parameters such as the amount of HS-CH3-IM, the sample volume, pH, and the shaking/centrifuging time were optimized. Under the optimal conditions, good linear range (LR), LOD, and enrichment factor (EF) were obtained 0.05–1.7 μg L−1, 15 ng L−1, and 19.82 respectively (RSD% < 1.45).
References
World Health Organization, Manganese in drinking-water: background document for development of WHO guidelines for drinking-water quality, World Health Organization, Geneva, 2004. https://apps.who.int/iris/handle/10665/75376.
J. Briffa, E. Sinagra, R. Blundell, Heavy metal pollution in the environment and their toxicological effects on humans, Heliyon, 6 (2020) e04691. https://doi.org/10.1016/j.heliyon.2020.e04691.
W. Feng, G. Mao, Q. Li, W. Wang, Y. Chen, T. Zhao, F. Li, Y. Zou, H. Wu, L. Yang, X. Wu, Effects of chromium malate on glycometabolism, glycometabolism-related enzyme levels and lipid metabolism in type 2 diabetic rats: A dose–response and curative effects study, J. Diabetes. Investig., 6 (2015) 396-407. https://doi.org/10.1111/jdi.12350.
A. Houldsworth, W. R, A. Fisher, D. A, A. Millward, Proposed Relationships between the degree of insulin resistance, serum chromium Level/BMI and renal function during pregnancy and the pathogenesis of gestational diabetes mellitus, Int. J. Endocrinol. Metab. Disord., 3 (2017)1-8. http://dx.doi.org/10.16966/2380-548X.132.
P. Baszuk, B. Janasik, S. Pietrzak, W. Marciniak, E. Reszka, K. Białkowska, E. Jabłońska, M. Muszyńska, M. Lesicka, R. Derkacz, T. Grodzki, J. Wójcik, M. Wojtyś, T. Dębniak, C. Cybulski, J. Gronwald, B. Kubisa, N. Wójcik, J. Pieróg, D. Gajić, P. Waloszczyk, R.J. Scott, W. Wąsowicz, A. Jakubowska, J. Lubiński, M.R. Lener, Lung cancer occurrence-correlation with serum chromium levels and genotypes, Biol. Trace Elem. Res., 199 (2021) 1228-1236. https://doi.org/10.1007/s12011-020-02240-6.
G.B. Post, Recent US state and federal drinking water guidelines for per- and polyfluoroalkyl substances, Environ. Toxicol. Chem., 40 (2021) 550-563. https://doi.org/10.1002/etc.4863.
American conference of governmental industrial hygienists, threshold limit values (ACGIH TLVs), new document for chemical substances, USA, 2018. https://www.acgih.org/science/tlv-bei-guidelines/.
National Institute for Occupational Safety and Health (NIOSH), occupational exposure to hexavalent chromium, centers for disease control and prevention, 2018. https://www.cdc.gov/niosh/topics/hexchrom/default.html.
R. Bevan, The first draft of the background document on chromium in drinking-water for the development of the WHO guidelines for drinking-water quality, World Health Organization. Geneva, 2019. https://www.who.int/water_sanitation_health/water-quality/guidelines/chemicals/draft-chromium-190924.pdf.
H. Peng, J. Guo, Removal of chromium from wastewater by membrane filtration, chemical precipitation, ion exchange, adsorption electrocoagulation, electrochemical reduction, electrodialysis, electrodeionization, photocatalysis and nanotechnology: a review, Environ. Chem. Lett., 18 (2020) 2055-2068. https://doi.org/10.1007/s10311-020-01058-x.
A. Mnif, I. Bejaoui, M. Mouelhi, B. Hamrouni, Hexavalent chromium removal from model water and car shock absorber factory effluent by nanofiltration and reverse osmosis membrane, Int. J. Anal. Chem., 2017 (2017) 7415708. https://doi.org/10.1155/2017/7415708.
D. Spanu, D. Monticelli, G. Binda, C. Dossi, L. Rampazzi, S. Recchia, One-minute highly selective Cr (VI) determination at ultra-trace levels: An ICP-MS method based on the on-line trapping of Cr (III), J. Hazard. Mater., 412 (2021) 125280. https://doi.org/10.1016/j.jhazmat.2021.125280.
N. Thị Hue, N. Van Hop, H. Thai Long, N. Hai Phong, T.H. Uyen, L. Quoc Hung, N. Nhi Phuong, Determination of chromium in natural water by adsorptive stripping voltammetry using in situ bismuth film electrode, J. Environ. Public Health, 2020 (2020) 1347836. https://doi.org/10.1155/2020/1347836.
N.A. Yazid, Y.C. Joon, Co-precipitation synthesis of magnetic nanoparticles for efficient removal of heavy metal from synthetic wastewater, AIP Conf. Proc., 2124 (2019) 020019. https://doi.org/10.1063/1.5117079.
E. Kazemi, A.M. Haji Shabani, S. Dadfarnia, F. Izadi, Speciation and determination of chromium ions by dispersive micro solid phase extraction using magnetic graphene oxide followed by flame atomic absorption spectrometry, Int. J. Environ. Anal. Chem., 97 (2017) 1080-1093. https://doi.org/10.1080/03067319.2017.1381693.
T.S. Munonde, N.W. Maxakato, P.N. Nomngongo, Preconcentration and speciation of chromium species using ICP-OES after ultrasound-assisted magnetic solid phase extraction with an amino-modified magnetic nanocomposite prepared from Fe3O4, MnO2 and Al2O3, Microchim. Acta, 184 (2017) 1223-1232. https://doi.org/10.1007/s00604-017-2126-2.
B.-H. Chen, S.-J. Jiang, A.C. Sahayam, Determination of Cr (VI) in rice using ion chromatography inductively coupled plasma mass spectrometry, Food. Chem., 324 (2020) 126698. https://doi.org/10.1016/j.foodchem.2020.126698.
D.J. Butcher, Recent highlights in graphite furnace atomic absorption spectrometry, Appl. Spectrosc. Rev., 52 (2017) 755-773. https://doi.org/10.1080/05704928.2017.1303504.
M.B. Arain, I. Ali, E. Yilmaz, M. Soylak, Nanomaterial's based chromium speciation in environmental samples: A review, TrAC, Trends Anal. Chem., 103 (2018) 44-55. https://doi.org/10.1016/j.trac.2018.03.014.
N. Campillo, P. Viñas, J. Šandrejová, V. Andruch, Ten years of dispersive liquid–liquid microextraction and derived techniques, Appl. Spectrosc. Rev., 52 (2017) 267-415. https://doi.org/10.1080/05704928.2016.1224240.
Y.-W. Wu, J. Zhang, J.-F. Liu, L. Chen, Z.-L. Deng, M.-X. Han, X.-S. Wei, A.-M. Yu, H.-L. Zhang, Fe3O4@ZrO2 nanoparticles magnetic solid phase extraction coupled with flame atomic absorption spectrometry for chromium (III) speciation in environmental and biological samples, Appl. Surf. Sci., 258 (2012) 6772-6776. https://doi.org/10.1016/j.apsusc.2012.03.057.
A. Saboori, A nanoparticle sorbent composed of MIL-101(Fe) and dithiocarbamate-modified magnetite nanoparticles for speciation of Cr (III) and Cr (VI) prior to their determination by electrothermal AAS, Microchim. Acta, 184 (2017) 1509-1516. https://doi.org/10.1007/s00604-017-2155-x.
K.M. Diniz, C.R.T. Tarley, Speciation analysis of chromium in water samples through sequential combination of dispersive magnetic solid phase extraction using mesoporous amino-functionalized Fe3O4/SiO2 nanoparticles and cloud point extraction, Microchem. J., 123 (2015) 185-195. https://doi.org/10.1016/j.microc.2015.06.011.
E. Yıldız, H. Çabuk, Dispersive liquid-liquid microextraction method combined with sugaring-out homogeneous liquid-liquid extraction for the determination of some pesticides in molasses samples, J. Sep. Sci., 44 (2021) 4151-4166. https://doi.org/10.1002/jssc.202100551.
F.C. Pinheiro, M.Á. Aguirre, J.A. Nóbrega, N. González-Gallardo, D.J. Ramón, A. Canals, Dispersive liquid-liquid microextraction based on deep eutectic solvent for elemental impurities determination in oral and parenteral drugs by inductively coupled plasma optical emission spectrometry, Anal. Chim. Acta, 1185 (2021) 339052. https://doi.org/10.1016/j.aca.2021.339052.
Copyright (c) 2022 Analytical Methods in Environmental Chemistry Journal

This work is licensed under a Creative Commons Attribution 4.0 International License.
JOURNAL PUBLISHING AGREEMENT
PLEASE PROVIDE US THE FOLLOWING INFORMATION,
Article entitled:
Corresponding author:
To be published in the journal:
Your Status
I am the sole author of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
I am one author signing on behalf of all co-authors of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
Please tick the above blanks (as appropriate), review the Journal Publishing Agreement, and then sign and date the document in black ink.
Published Journal Article: the author may share a link to the formal publication through the relevant DOI. Additionally theses and dissertations which contain embedded Published Journal Articles as part of the formal submission may be hosted publicly by the awarding institution with a link to the formal publication through the relevant DOI. Any other sharing of Published Journal Articles is by agreement with the publisher only.
Signed: ______________________________________ Name printed: ___________________________________________
Title and Company (if employer representative): _______________________Date: __________________________________