Determination of mercury values in urine and air of chloralkali workers by copper nanoparticles functionalized in carboxylic carbon nanotubes and the effects of mercury exposure on oxidative stress
Volume 5, Issue 02, Pages 76-89, Jun 2022 *** Field: Analytical Method in Environment and human
Abstract
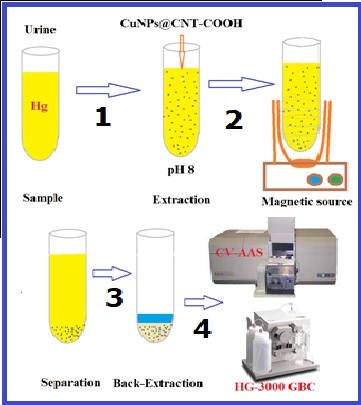
Mercury exposure can produce toxic organic compounds in the body. Also, mercury can potentially cause oxidative damage and cellular disorders. In this study, the determination of mercury values in urine and air of chloralkali workers based on copper nanoparticles functionalized in carboxylic carbon nanotubes (CuNPs@CNT-COOH) were obtained by cold vapor atomic absorption spectrometer (CV-AAS). The urine samples were determined by magnetic solid-phase extraction (MSPE) at pH 8.0. By measuring the mercury level in the air and the urine sample of workers, the level of oxidative stress (Malondialdehyde (MDA), Superoxide Dismutase (SOD) and Catalase (Cat)), Interleukin-6 (IL-6), and Tumor Necrosis Factor α (TNF-α) as the proinflammatory cytokines were measured in the subject group. The results revealed statistically significant differences in the mercury level of the urine samples in the case and control groups (p<0.001). Similarly, the malondialdehyde (MDA) level was significantly different between the two research groups (p<0.001). Catalase concentration was not significantly different in the two groups (p=0.059). The LOD and linear range for mercury determination in urine were achieved at 0.012 µg L−1 and 0.05-7.0 µg L−1, respectively. Workers’ exposure to mercury can significantly increase oxidative stress and inflammatory cell signaling molecules such as cytokines.
References
T. Mihaiescu, R. Mihaiescu, A. Odagiu, Environmental issues within the Chlor-alkali manufacturing industry-mercury cell process. Bull. Univ. Agric. Sci. Vet. Med., 69 (2012) 168-176. https://doi.org/10.15835/buasvmcn-agr:8746
D. Soleymani-ghoozhdi, R. Parvari, Y. Jahani, M. Mehdipour-Raboury, A. Faghihi-Zarandi, A new analytical method based on Co-Mo nanoparticles supported by carbon nanotubes for removal of mercury vapor from the air by the amalgamation of solid-phase air removal, Anal. Methods Environ. Chem. J., 50.1 (2022) 22-35. https://doi.org/10.24200/amecj.v5.i01.163
F. Golbabai, A. Ebrahimi, H. Shirkhanloo, M.R. Baneshi, A. Faghihi Zarandi, M.G. Kian, Performance comparison survey of multi-walled and single-walled carbon nanotubes for adsorption and desorption of Mercury Vapors in the air. Iran Occupational Health, 10 (2013) 20-31. http://ioh.iums.ac.ir/article-1-813-en.html
G.A. Wiggers, F.M. Peçanha, A. M. Briones, J.V. Perez-Giron, M. Miguel, D.V. Vassallo, Low mercury concentrations cause oxidative stress and endothelial dysfunction in conductance and resistance arteries, Am. J. Physiol. Heart Circ. Physiol., 295 (2008) H1033-H21043. https://doi.org/10.1152/ajpheart.00430.2008
I. Garcia-Herrero, M. Margallo, R. Onandía, R. Aldaco, A. Irabien, Environmental challenges of the chlor-alkali production: Seeking answers from a life cycle approach, Sci. Total Environ., 580 (2017)147-157. https://doi.org/10.1016/j.scitotenv.2016.10.202
G. Bjørklund, M. Dadar, J. Mutter, J. Aaseth, The toxicology of mercury, current research and emerging trends, Environ. Res., 159 (2017) 545-554. https://doi.org/10.1016/j.envres.2017.08.051
R.M. Gardner, J.F. Nyland, I. A. Silva, A.M. Ventura, J.M. de Souza, E.K. Silbergeld, Mercury exposure, serum antinuclear/antinucleolar antibodies, and serum cytokine levels in mining populations in Amazonian Brazil, a cross-sectional study, Environ. Res., 110 (2010) 345-354. https://doi.org/10.1016/j.envres.2010.02.001
D. Winalski, S. Mayson, J. Savitz, Poison plants, chlorine factories are a major global source of mercury, Oceana publisher, (2005). https://oceana.org/wp-content/uploads/sites/18/poisonplants2FINAL.pdf
J.F. Risher, H.E. Murray, G.R. Prince, Organic mercury compounds, human exposure and its relevance to public health, Toxicol. Ind. Health, 18 (2002) 109-160. https://doi.org/ 10.1191/0748233702th138oa
J.F. Risher, R.A. Nickle, S.N. Amler, Elemental mercury poisoning in occupational and residential settings, Int. J. Hyg. Environ. Health, 206 (2003) 371-379. https://doi.org/10.1078/1438-4639-00233
E.G. Pacyna, J. Pacyna, K. Sundseth, J. Munthe, K. Kindbom, S. Wilson, Global emission of mercury to the atmosphere from anthropogenic sources in 2005 and projections to 2020, Atm. Environ., 44 (2010) 2487-2499. https://doi.org/10.1016/j.atmosenv.2009.06.009
S.Y. Do, C.G. Lee, J.Y. Kim, Y.H. Moon, M.S. Kim, I.H. Bae, Cases of acute mercury poisoning by mercury vapor exposure during the demolition of a fluorescent lamp factory, Annals Occup. Environ. Med., 29 (2017) 1-8. https://doi.org/10.1186/s40557-017-0184-x
L.C. Curlin, T.V. Bommaraju, C. B. Hansson, Alkali and chlorine products, chlorine and sodium hydroxide, Kirk‐Othmer encyclopedia of chemical technology, (2000). https://doi.org/10.1002/0471238961.0308121503211812.a01
J. Crook, A. Mousavi, The chlor-alkali process, A review of history and pollution, Environ. Forensics, 17 (2016) 211-217. https://doi.org/10.1080/15275922.2016.1177755
D.G. Ellingsen, R. Bast-Pettersen, J. Efskind, Y. Thomassen, Neuropsychological effects of low mercury vapor exposure in chloralkali workers, Neurotoxicol., 22 (2001) 249-258. https://doi.org/10.1590/S0100-879X2007000300019
S. Hirano, Evaluation of pulmonary toxicity of heavy metal compounds, Japan. J. Hyg., 50 (1996) 1013-1025. https://doi.org/doi: 10.3233/WOR-203194.
T. Takahashi, M. Fujimura, M. Koyama, M. Kanazawa, F. Usuki, M. Nishizawa, Methylmercury causes blood-brain barrier damage in rats via upregulation of vascular endothelial growth factor expression, PLOS One, 12 (2017) e0170623. https://doi.org/10.1371/journal.pone.0170623
C. Bottino, M. Vázquez, V. Devesa, U. Laforenza, Impaired aquaporins expression in the gastrointestinal tract of rat after mercury exposure, J. Appl. Toxicol., 36 (2016) 113-120. https://doi.org/10.1002/jat.3151
Y.S. Lin, G. Ginsberg, J.W. Lin, B. Sonawane, Mercury exposure and omega-3 fatty acid intake in relation to renal function in the US population, Int. J. Hyg. Environ. Health, 214 (2014) 465-472. https://doi.org/10.1016/j.ijheh.2013.09.004
J. Anglen, S.E. Gruninger, H.N. Chou, J. Weuve, M.E. Turyk, S. Freels, Occupational mercury exposure in association with prevalence of multiple sclerosis and tremor among US dentists, J. Am. Dental Assoc., 146 (2015) 659-668. https://doi.org/10.1016/j.adaj.2015.05.016
R. Zefferino, C. Piccoli, N. Ricciardi, R. Scrima, N. Capitanio, Possible mechanisms of mercury toxicity and cancer promotion, Involvement of gap junction intercellular communications and inflammatory cytokines, Oxid. Med. Cell. Longev., 2017 (2017) 7028583. https://doi.org/10.1155/2017/7028583
M. Neghab, M. Amin Norouzi, A. Choobineh, M. Reza Kardaniyan, J. Hassan Zadeh, Health effects associated with long-term occupational exposure of employees of a chlor-alkali plant to mercury, Int. J. Occup. Safe. Ergonomics, 18 (2012) 97-106. https://doi.org/ 10.1080/10803548.2012.11076920
Z. Ahmadi, A. Moradabadi, D. Abdollahdokht, M. Mehrabani, M.H. Nematollahi, Association of environmental exposure with hematological and oxidative stress alteration in gasoline station attendants, Environ. Sci. Pollut. Res., 26 (2019) 20411-20417. Environ. Sci. https://doi.org/10.1007/s11356-019-05412-7
D. Soleymani, S. Zargari, A. Faghihi-Zarandi, Separation and determination of mercury from nail and hair in petrochemical workers based on silver carbon nanotubes by microwave-assisted headspace sorbent trap, Anal. Methods Environ. Chem. J., 3 (2020) 21-33. https://doi.org/10.24200/amecj.v3.i02.99
B. Elsebai, M.E. Ghica, M.N. Abbas, C.M. Brett, Catalase based hydrogen peroxide biosensor for mercury determination by inhibition measurements, J. Hazard. Mater., 340 (2017) 344-350. https://doi.org/10.1016/j.jhazmat.2017.07.021
O. Ighodaro, O. Akinloye, First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid, Alexandria J. Med., 54 (2018) 287-293. https://doi.org/10.1016/j.ajme.2017.09.001
B.B. Gump, E. Gabrikova, K. Bendinskas, A.K. Dumas, C.D. Palmer, P.J. Parsons, Low-level mercury in children: associations with sleep duration and cytokines TNF-α and IL-6, Environ. Res., 134 (2014) 228-232. https://doi.org/10.1016/j.envres.2014.07.026
E.E. Kwaansa-Ansah, E.K. Armah, F. Opoku, Assessment of total mercury in hair, urine and fingernails of small–scale gold miners in the Amansie West District, Ghana. J. Health Pollut., 9 (2019)190306. https://doi.org/10.5696/2156-9614-9.21.190306
G.F. Templeton, A two-step approach for transforming continuous variables to normal: implications and recommendations for IS research, Commun. Assoc. Inf. Syst., 28 (2011) 4. https://doi.org/10.17705/1cais.02804
M. Azami, A. Mansouri, M. Khataee, A. Soleymani, K. Sayehmiri, A systematic review and meta-analysis of mercury concentrations in blood, urine, and area air samples among dentists in Iran, J. Maz. Univ. Med. Sci., 27.152 (2017) 203-216. http://jmums.mazums.ac.ir/article-1-8036-en.html
A.C.B.A. Lopes, M.R. Urbano, A. de Souza-Nogueira, G.H. Oliveira-Paula, A.P. Michelin, H.C. Maria de Fátima, Association of lead, cadmium and mercury with paraoxonase activity and malondialdehyde in a general population in Southern Brazil, Environ. Res., 156 (2017) 674-682. https://doi.org/10.1016/j.envres.2017.04.036
M. Mahboob M, Shireen KF, Atkinson A, Khan AT, Lipid peroxidation and antioxidant enzyme activity in different organs of mice exposed to low level of mercury, Journal of Environmental Science and Health, Part B, 36.5 (2001) 687-697. https://doi.org/10.1081/PFC-100106195
H.F. Al-azzawie, A. Umran, N.H. Hyader, Oxidative stress, antioxidant status and DNA damage in a mercury exposure workers, Br. J. Pharmacol. Toxicol., 4 (2013) 80-88. https://doi.org/10.19026/bjpt.4.5367
M. Jozefczak, T. Remans, J. Vangronsveld, A. Cuypers, Glutathione is a key player in metal-induced oxidative stress defenses, Int. J. Mol. Sci., 13 (2012) 3145-3175. https://doi.org/10.3390/ijms13033145
E.A. Belyaeva, T.V. Sokolova, L.V. Emelyanova, I.O. Zakharova, Mitochondrial electron transport chain in heavy metal-induced neurotoxicity: effects of cadmium, mercury, and copper, Sci. World J., 2012 (2012)136063. https://doi.org/10.1100/2012/136063
V.L. Cariccio, A. Samà, P. Bramanti, E. Mazzon, Mercury involvement in neuronal damage and in neurodegenerative diseases, Biol. Trace Elem. Res., 187 (2019) 341-356. https://doi.org/10.1007/s12011-018-1380-4
L. Bagenstose, P. Salgame, M. Monestier, Mercury-induced autoimmunity in the absence of IL, Clin. Exp. Immunol., 114 (1998) 9. https://doi.org/10.1046/j.1365-2249.1998.00704.x
S.H. Kim, V.J. Johnson, R.P. Sharma, Mercury inhibits nitric oxide production but activates proinflammatory cytokine expression in murine macrophage: differential modulation of NF-κB and p38 MAPK signaling pathways, Nitric Oxide, 7 (2002) 67-74. https://doi.org/10.1016/s1089-8603(02)00008-3
J.F. Nyland, D. Fairweather, D.L. Shirley, S.E. Davis, N.R. Rose, E.K. Silbergeld, Low-dose inorganic mercury increases severity and frequency of chronic coxsackievirus-induced autoimmune myocarditis in mice, Toxicol. Sci., 125 (2012) 134-143. https://doi.org/10.1093/toxsci/kfr264
R.M. Gardner, J.F. Nyland, S.L. Evans, S.B. Wang, K.M. Doyle, C.M. Crainiceanu, Mercury induces an unopposed inflammatory response in human peripheral blood mononuclear cells in vitro, Environ. Health Perspect., 117 (2009)1932-1938. https://doi.org/10.1289/ehp.0900855
ESM*******************************************************************************************
R.N. Monastero, R. Karimi, J.F. Nyland, J. Harrington, K. Levine, J.R. Meliker, Mercury exposure, serum antinuclear antibodies, and serum cytokine levels in the long Island study of seafood consumption, A cross-sectional study in NY, USA, Environ. Res., 156 (2017) 334-340. https://doi.org/10.1016/j.envres.2017.03.037
F. Golbabaei, A. Ebrahimi, Single-walled carbon nanotubes (SWCNTs), as a novel sorbent for determination of mercury in air, Global J. Health Sci., 8 (2016) 273. https://doi.org/10.5539/gjhs.v8n7p273
Copyright (c) 2022 Analytical Methods in Environmental Chemistry Journal

This work is licensed under a Creative Commons Attribution 4.0 International License.
JOURNAL PUBLISHING AGREEMENT
PLEASE PROVIDE US THE FOLLOWING INFORMATION,
Article entitled:
Corresponding author:
To be published in the journal:
Your Status
I am the sole author of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
I am one author signing on behalf of all co-authors of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
Please tick the above blanks (as appropriate), review the Journal Publishing Agreement, and then sign and date the document in black ink.
Published Journal Article: the author may share a link to the formal publication through the relevant DOI. Additionally theses and dissertations which contain embedded Published Journal Articles as part of the formal submission may be hosted publicly by the awarding institution with a link to the formal publication through the relevant DOI. Any other sharing of Published Journal Articles is by agreement with the publisher only.
Signed: ______________________________________ Name printed: ___________________________________________
Title and Company (if employer representative): _______________________Date: __________________________________