Determination of tetrafluoroborate in wastewaters by ion chromatography after ion pair liquid-liquid dispersive microextraction
Volume 5, Issue 04, Pages 77-86, Dec 2022 *** Field: Analytical Chemistry
Abstract
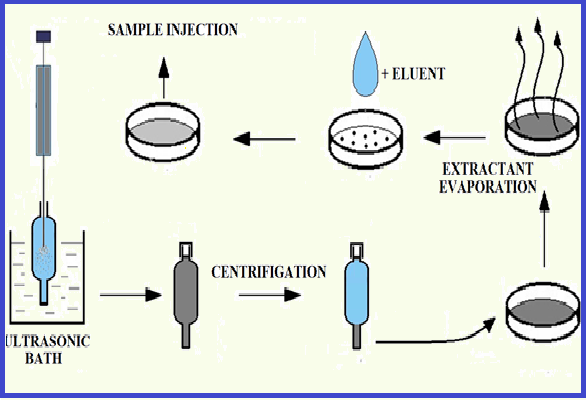
The ion chromatographic method was developed to determine tetrafluoroborate ion (BF4-) in different types of water using ion pair liquid-liquid dispersive microextraction. Tetrafluoroborate was extracted into an organic phase (1,2-dichloroethane) as an ion pair with a tetrabutylammonium cation (TBA+). The most complete formation of [(ТBА+)(BF4-)] was observed at ion-pair reagent concentration of at least 5 mmol L-1 (C(BF4-) ≤ 1mg L-1). Ultrasonic irradiation was used to disperse the extractant. The achieved concentration factor (K) was 29±3, and the degree of extraction (R) was 50±5%. The limit of detection of tetrafluoroborate using the microextraction technique was 7×10-3 mg L-1. The method applies to the analysis of different water origins. The presence of the main contained anions does not interfere with the microextraction and chromatographic determination of tetrafluoroborate. The maximum molar ratio of BF4- to diverse ions is 1:104 for fluoride, chloride, bromide, nitrate ions, and 1:102 for sulfate and perchlorate ions.
References
P.T. Anastas, Green chemistry and the role of analytical methodology development, Crit. Rev. Anal. Chem., 29 (1999) 167–175. https://doi.org/10.1080/10408349891199356
M. Koel, M. Kaljurand, Application of the principles of green chemistry in analytical chemistry, Pure Appl. Chem., 78 (2006) 1993–2002. https://doi.org/10.1351/pac200678111993
S. Armenta, S. Garrigues, M. de la Guardia, Green Analytical Chemistry, TrAC-Trends Anal. Chem., 27 (2008) 497–511. https://doi.org/10.1016/j.trac.2008.05.003
B.A. de Marco, B.S. Rechelo, E.G. Tótoli, A.C. Kogawa, H.R.N. Salgado, Evolution of green chemistry and its multidimensional impacts: A review, Saudi Pharm. J., 27 (2019) 1–8. https://doi.org/10.1016/j.jsps.2018.07.011
R. Goutham, P.Rohit, S.S. Vigneshwar, A. Swetha, J. Arun, K.P. Gopinath, A. Pugazhendhi, Ionic liquids in wastewater treatment: A review on pollutant removal and degradation, recovery of ionic liquids, economics and future perspectives, J. Mol. Liq., 349 (2021) 118150. https://doi.org/10.1016/j.molliq.2021.118150
M. Khraisheh, F. AlMomani, M. Inamdar, M.K. Hassan, M.A. Al-Chouti, Ionic liquids application for wastewater treatment and biofuel production: A mini review, J. Mol. Liq., 337 (2021) 116421. https://doi.org/10.1016/j.molliq.2021.116421
H. Kim, S. Baek, T. Lim, J.J. Kim, Electrochemical reduction of nitrous oxide in 1-butyl-3-methylimidazolium tetrafluoroborate ionic liquid electrolyte, Electrochem. Commun., 113 (2020) 106688. https://doi.org/10.1016/j.elecom.2020.106688
A.N. Araújo, M.B. Etxebarria, J.L.F.C. Lima, M.C.B.S.M. Montenegro, R. Pérez Olmos, Tubular detectors for flow-injection potentiometric determination of tetrafluoroborate in electroplating baths, Anal. Chim. Acta, 293 (1994) 35-41. https://doi.org/10.1016/0003-2670(94)00073-5
Y. Dong, X. Liang, H. Yuan, S. Qi, F. Chen, D. Wang, Potential green fungicide: 16-oxo-1-oxa-4-azoniacyclohexadecan-4-ium tetrafluoroborate, Green Chem., 10 (2008) 990–994. https://doi.org/10.1039/b805797d
R. Biczak, Quaternary ammonium salts with tetrafluoroborate anion: Phytotoxicity and oxidative stress in terrestrial plants, J. Hazard. Mater., 304 (2016) 173-185. https://doi.org/10.1016/j.jhazmat.2015.10.055
L. Rusnakova, V. Andruch, I.S. Balogh, J. Škrlíková, A dispersive liquid-liquid microextraction procedure for determination of boron in water after ultrasound-assisted conversion to tetrafluoroborate, Talanta, 85 (2011) 541-545. https://doi.org/10.1016/j.talanta.2011.04.030
A. Bayati-Comitaki, M. M. Zahedi, Ultrasound-assisted formation of tetrafluoroborate: methylene blue for microextraction and flow based spectrophotometric determination of the boron, Int. J. Environ. Sci. Technol., 19 (2022) 10869–10876. https://doi.org/10.1007/s13762-022-04278-6
I. Kasahara, S. Hosokawa, N. Hata, S. Taguchi, K. Goto, Selective and sensitive spectrophotometric determination of tetrafluoroborate in wastewater after ion-pair extraction using bis[2-(5-chloro-2-pyridylazo)-5-diethylaminophenolato]cobalt(11a1s) a counter ion, Analyst, 118 (1993) 1205-1208. https://doi.org/10.1039/AN9931801205
S. Zhou, H. Yu, L. Yang, H. Ai, Fast determination of tetrafluoroborate by high-performance liquid chromatography using a monolithic column, J. Chromatogr. A., 1206 (2008) 200-203. https://doi.org/10.1016/j.chroma.2008.08.048
J. Katagiri, T.Yoshioka, T.Mizoguchi, Basic study on determination of total boron by conversion to tetrafluoroborate ion (BF4-) followed by ion chromatography, Anal. Chim. Acta, 570 (2006) 65–72. https://doi.org/10.1016/j.aca.2006.03.084
N. Phadungcharoen, N. Pengwanput, A. Nakapan, U. Sutitaphan, P. Thanomkloma, N. Jongudomsombuta, A. Chinsriwongkulb, T. Rojanarata, Ion pair extraction coupled with digital image colorimetry as a rapid and green platform for pharmaceutical analysis: an example of chlorpromazine hydrochloride tablet assay, Talanta, 219 (2020) 121271. https://doi.org/10.1016/j.talanta.2020.121271
M.L. Magnuson, E.T. Urbansky, C.A. Kelty, Determination of perchlorate at trace levels in drinking water by ion-pair extraction with electrospray ionization mass spectrometry, Anal. Chem. 72 (2000) 25-29. https://doi.org/10.1021/ac9909204
M. M. Baiser, H. Lund, Organic Electrochemistry: An Introduction and a Guide, M. Dekker, New York, J. Polym. Sci.: Polymer Letters Edition, 22 (1983) 459. https://doi.org/10.1002/pol.1984.130220809
A. Nemodruk, Z. Karalova, Analiticheskaya khimiya bora [Boron analitycal chemistry], Nauka, Moscow, 1964. https://agris.fao.org/agris-search/search.do?recordID=US201300591908
Me. G. Ryss, Khimiya ftora i ego neorganicheskikh soedinenij [The chemistry of fluorine and its inorganic compounds], Goshimizdat, Moscow, 1956. https://www.osti.gov/etdeweb/biblio/1512919
V.S. Shmidt, S.D. Nikitin, Regularities in interfacial tension and emulsion unmixing for solvent-extraction systems, At. Energ., 60 (1986) 467–475. https://doi.org/10.1007/BF01124091
K. Rajhardt, Rastvoriteli i effekty sredy v organicheskoj khimii [Solvents and solvent effects in organic chemistry], Mir Publ., Moscow, 1991. https://www.bestbookcentre.com/books/russian-books-mir-publishers-moscow
E.А. Mezhov, Ekstrakciya aminami i chetvertichnymi ammonievymi osnovaniyami [Extraction with amines and quaternary ammonium bases], Energoatomizdat Publ., Moscow, 1999. https://openlibrary.org/publishers/Energoatomizdat
L.P. Eksperiandova, K.N. Belikov, S.V. Khimchenko, T.A. Blank, Once again about the limits of detection and determination, J. Anal. Chem., 65 (2010) 229–234. https://doi.org/10.1134/S1061934810030020
Copyright (c) 2022 Analytical Methods in Environmental Chemistry Journal

This work is licensed under a Creative Commons Attribution 4.0 International License.
JOURNAL PUBLISHING AGREEMENT
PLEASE PROVIDE US THE FOLLOWING INFORMATION,
Article entitled:
Corresponding author:
To be published in the journal:
Your Status
I am the sole author of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
I am one author signing on behalf of all co-authors of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
Please tick the above blanks (as appropriate), review the Journal Publishing Agreement, and then sign and date the document in black ink.
Published Journal Article: the author may share a link to the formal publication through the relevant DOI. Additionally theses and dissertations which contain embedded Published Journal Articles as part of the formal submission may be hosted publicly by the awarding institution with a link to the formal publication through the relevant DOI. Any other sharing of Published Journal Articles is by agreement with the publisher only.
Signed: ______________________________________ Name printed: ___________________________________________
Title and Company (if employer representative): _______________________Date: __________________________________