Determination and prediction of peptide mobilities by micellar electro-kinetic chromatography using adaptive neuro-fuzzy inference system as a feature selection method
Vol 3, Issue 02, Pages 5-20,*** Field: Chemometrics in Analytical Chemistry
Abstract
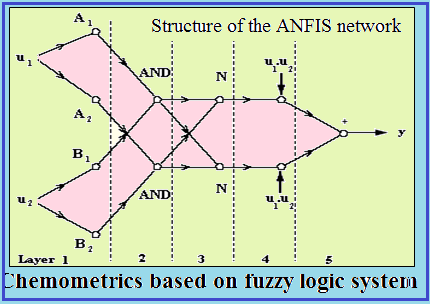
Mobility of 128 peptides composed of up to 14 amino acids is determined for sodium dodecyl sulfate (SDS) micellar systems using micellar electrokinetic chromatography (MEKC). The mobilities of these peptides are predicted using back propagation of error artificial neural networks (BP-ANNs). Adaptive neuro-fuzzy inference system (ANFIS) which can deal with linear and nonlinear phenomena is used to select the inputs of BP-ANN. A 3:4:1 BP-ANN model with four variables of Kappa substituent constant, Kappa(H), number of peptide bonds, (ln(N), molar refractivity of C-terminal, MRC, and steric effects at N-terminal, ES,N, which incorporate substituent, steric and molar refractivity effects as its inputs was developed. Comparison of Multiple Linear Regression (MLR) and ANN results shows the nonlinear characteristic of the phenomena. The nonlinear model was successful in predicting the mobilities of 120 peptides except for the ones (8 peptides) with negatively charged amino acids.
References
A.H. Rageh, U. Pyell, Pseudostationary ion-exchanger” sweeping as an online enrichment technique in teh determination of nucleosides in urine via micellar electrokinetic chromatography, Chromatogra., 82 (2019) 325–345.
A.H. Rageh, U. Pyell, Imidazolium-based ionic liquid-type surfactant as pseudostationary phase in micellar electrokinetic chromatography of highly hydrophilic urinary nucleosides, J Chromatogr., A 1316 (2013)135–146.
R.B. Yu, J. P. Quirino, Chiral Selectors in capillary electrophoresis: trends during 2017–2018, Molecules, 24 (2019) 1135.
J. Fiori, B. Pasquini, C. Caprini, S. Orlandini, S. Furlanetto, R. Gotti, Chiral analysis of theanine and catechin in characterization of green tea by cyclodextrin-modified micellar electrokinetic chromatography and high performance liquid chromatography, J. Chromatogr. A, 1562 (2018) 115–122.
Y. Liu, X. Wang, Enantioseparation of ofloxacin and its four related substances with ligand exchange–micellar electrokinetic chromatography using copper (II)-L-isoleucine complex as chiral selector, Chirality., 29 (2017) 422–429.
I.J. Stavrou, E.A. Agathokleous, C.P. Kapnissi-Christodoulou, Chiral selectors in CE: Recent development and applications, Electrophoresis, 38 (2017) 786–819.
V. Patel , S. A. Shamsi, Carbohydrate based polymeric surfactants for chiral micellar electrokinetic chromatography (CMEKC) coupled to mass spectrometry, Methods Mol. Biol., 1985 (2019) 417–444.
Y. Liu, S.A. Shamsi, Development of novel micellar electrokinetic chromatography mass spectrometry for simultaneous enantioseparation of venlafaxine and dimethyl-venlafaxine: Application to analysis of drug-drug interactions, J. Chromatogr. A, 1420 (2015) 119–128.
R. L. C. Voeten, I. K. Ventouri, R. Haselberg, G. W. Somsen, Capillary electrophoresis: trends and recent Advances, Anal Chem., 90 (2018) 1464–1481.
C.-R. Chung, J.-H. Jhong, Z. Wang, S. Chen, Y. Wan, Characterization and identification of natural antimicrobial peptides on different organisms, Int. J. Mol. Sci., 21(2020) 986. doi:10.3390/ijms21030986
Fausett, L, Fundamentals of neural networks, architectures, algorithms, and applications, Prentice-Hall, inc., New Jersey, 1994.
M. Sugimoto, S. Kikuchi, M. Arita, T. Soga, T. Nishioka, M. Tomita, Large-scale prediction of cationic metabolite identity and migration time in capillary electrophoresis mass spectrometry using artificial neural networks, Anal. Chem., 77 (2005) 78-84
K. Shinoda, M. Sugimoto, N. Yachie, N. Sugiyama, T. Masuda, M. Robert, T Soga, M. Tomita, Prediction of liquid chromatographic retention times of peptides generated by protease digestion of the Escherichia coli proteome using artificial neural networks, J. Proteome Res., 5 (2006) 3312-3317.
K. Khan, K. Roy, Ecotoxicological modelling of cosmetics for aquatic organisms: A QSTR approach, SAR QSAR Environ. Res., 28 (2017) 567-594.
F. Yang, J. Tian, Y. Xiang, Z. Zhang, P. de B. Harrington, Near infrared spectroscopy combined with least squares support vector machines and fuzzy rule-building expert system applied to diagnosis of endometrial carcinoma, Cancer Epidemiol., 36 (2012) 317-323.
R. Darnag, B.Minaoui, M. Fakir, QSAR models for prediction study of HIV protease inhibitors using support vector machines, neural networks and multiple linear regression, Arab. J. Chem., (2017) S600-S608.
G.J. Klir, B.Yuan, Fuzzy sets and fuzzy logic, theory and applications. Prentice-Hall, inc., New Jersey, 1995.
H. Zarei, A. Khastan, A. Zafar, Optimal control of linear fuzzy time-variant controlled systems, Iran. J. Fuzzy Sys., 17 (2020) 1-12.
J. Jantzen, Neurofuzzy modeling technical report no 98-H-874, 1998.
M. G. Khaledi, Micellar electrokinetic chromatography in high performance capillary electrophoresis: theory, technique and applications, Wiley, New York, 1998.
Fuzzy toolbox; Copyright 1994-2002, the math works, Inc. revision: 1.8; Roger Jang, Aug. 1997.
J. Liu, H. Wang, LingZhi oligopeptides amino acid sequence analysis and anticancer potency evaluation, RSC Adv., 10 (2020) 8377-8384.
C. Hansch, A. Leo, D. Hoekman, Exploring QSAR, hydrophobic, electronic, and steric constants, ACS, Washington DC., 1995.
R. W. Taft, Steric effects in organic chemistry, John Wiley and Sons Inc.; New York, 1956.
S. S. Samanta, S. P. Roche, Synthesis and reactivity of α‐haloglycine esters: hyperconjugation in action, Eur. J. Inorg. Chem., 2019 (2019) 6597-6605.
J.A. Macphee, A. Panaye, J.E. Dubois, Steric effects-I: A critical examination of the taft steric parameter-Es, definition of a revised, broader and homogeneous scale, extension to highly congested alkyl groups, Tetrahedron., 34 (1978) 3553-3562.
Copyright (c) 2020 Analytical Methods in Environmental Chemistry Journal

This work is licensed under a Creative Commons Attribution 4.0 International License.
JOURNAL PUBLISHING AGREEMENT
PLEASE PROVIDE US THE FOLLOWING INFORMATION,
Article entitled:
Corresponding author:
To be published in the journal:
Your Status
I am the sole author of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
I am one author signing on behalf of all co-authors of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
Please tick the above blanks (as appropriate), review the Journal Publishing Agreement, and then sign and date the document in black ink.
Published Journal Article: the author may share a link to the formal publication through the relevant DOI. Additionally theses and dissertations which contain embedded Published Journal Articles as part of the formal submission may be hosted publicly by the awarding institution with a link to the formal publication through the relevant DOI. Any other sharing of Published Journal Articles is by agreement with the publisher only.
Signed: ______________________________________ Name printed: ___________________________________________
Title and Company (if employer representative): _______________________Date: __________________________________