A new kinetic models analysis for CO adsorption on palladium zeolite nanostructure by roll-coating technique
Volume 3, Issue 02, Pages 92-107, Jun 2020 *** Field: Environmental Analytical Chemistry
Abstract
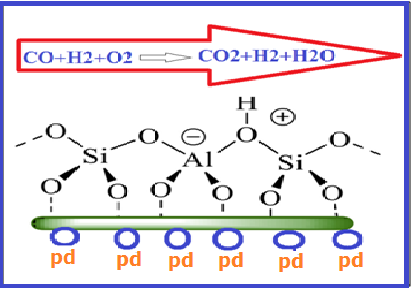
The aim of this article was the fabrication of Al2O3/Pd(NO3)2/zeolite adsorbent through roll-coating technique for CO gas adsorption. Transmission electron microscopy (TEM), field-emission scanning electron microscopy (FESEM), X-ray diffraction (XRD), and energy-dispersive x-ray spectroscopy (EDX) were performed to investigate the morphological, structural, and elemental properties of Al2O3/Pd(NO3)2/zeolite adsorbent. A continuous gas analyzer KIMO KIGAZ 210 was applied for testing CO gas adsorption on as-present adsorbent in an experimental set-up. The calculated amounts of adsorption capacity at equilibrium time was 111.16 mg g-1 according to the previous published article. The Elovich, Avrami, and Fractional power kinetic models were studied for this adsorbent. The equal value of experimental and theoretical adsorption capacity at equilibrium time as well as the unit value of regression coefficient indicate that Avrami kinetic model was a suitable model to describe Co removal through Al2O3/Pd(NO3)2/zeolite adsorbent.
References
J. Cleland, World population growth; past, present and future, Environ. Res. Econ., 55 (2013) 543–554.
M. Hussain, G. Liu, B. Yousaf, R. Ahmed, F. Uzma, M. U. Ali, H. Ullah, A. R. Butt, Regional and sectoral assessment on climate-change in Pakistan: Social norms and indigenous perceptions on climate-change adaptation and mitigation in relation to global context, J. Clean. Prod., 200 (2018) 791–808.
S, Mahajan. S, Jagtap, Metal-oxide semiconductors for carbon monoxide (CO) gas sensing. Materials. Rev., 18 (2020) 100483.
C. Qin, B. Wang, N. Wu, C. Han, C. Wu, X. Zhang, Q. Tian, S. Shen, P. Li, Y. Wang, Metal-organic frameworks derived porous Co3O4 dodecahedrons with abundant active Co3+ for ppb-level CO gas sensing, Appl. Surf. Sci., 506 (2019) 144900.
ACGIH Chemical Substances TLV Committee, Notice of intended change - carbon monoxide, Appl. Occup. Environ. Hyg., 6 (1991) 621–624.
A. M. Awad, R. Jalab, A. Benamor, M. S. Naser, M. M. Ba-Abbad, M. El-Naas, A. W. Mohammad, Adsorption of organic pollutants by nanomaterial-based adsorbents: An overview. J. Mol. Liq., 301 (2019) 112-335.
M. N. Rashed, Adsorption technique for the removal of organic pollutants from water and wastewater, Organic Pollutants-Monitoring, Risk and Treatment, Intech. Open, (2013) 167-194.
P. K. Gautam, A. Singh, K. Misra, A. K. Sahoo, S. K. Samanta, Synthesis and applications of biogenic nanomaterials in drinking and wastewater treatment, J. Environ. Manage., 231 (2019) 734–748.
C. Santhosh, V. Velmurugan, G. Jacob, S. K. Jeong, A. N. Grace, A. Bhatnagar, Role of nanomaterials in water treatment applications: A review, Chem. Eng. J., 306 (2016) 1116– 1137.
Y. Zhang, B. Wu, H. Xu, H. Liu, M. Wang, Y. He, B. Pan, Nanomaterials-enabled water and wastewater treatment, Nano Impact, 3 (2016) 22–39.
R. Zhao, S. Deng, S. Wang, L. Zhao, Y. Zhang, B. Liu, H. Li, Z. Yu, Thermodynamic research of adsorbent materials on energy efficiency of vacuum pressure swing adsorption cycle for CO capture, Appl. Thermal Eng., 128 (2018) 818–829.
K. T. Chue, J. N. Kim, Y. J. Yoo, S. H. Cho, R. T. Yang, Comparison of activated carbon and zeolite 13X for CO2 recovery from flue gas by pressure swing adsorption, Ind. Eng. Chem. Res., 2 (1995) 591– 598.
R, Krishna, Adsorptive separation of CO2/CH4/CO gas mixtures at high pressures, Micropor. Mesopor. Mater., 156 (2012) 217–223.
M. Clausse, J. Bonjour, F. Meunier, Adsorption of gas mixtures in TSA adsorbers under various heat removal conditions, Chem. Eng. Sci., 17 (2004) 3657–3670.
J.-R. Li, R. J. Kuppler, H. C. Zhou, Selective gas adsorption and separation in metal–organic frameworks, Chem. Soc. Rev., 5 (2009) 1477.
M., Bui, C. S. Adjiman, A. Bardow, E. J. Anthony, A. Boston, S. Brown, et. Al., Carbon capture and storage (CCS): the way forward, Energ. Environ. Sci., 5 (2018) 1062–1176.
A. Samanta, A. Zhao, G. K. H. Shimizu, P. Sarkar, R. Gupta, Post combustion CO2 capture using solid sorbents: a review, Ind. Eng. Chem. Res., 4 (2011) 1438–1463.
D. Lozano-Castelló, D. Cazorla-Amorós, A. Linares-Solano, D. F. Quinn, Activated carbon monoliths for methane storage: influence of binder, Carbon, 15 (2002) 2817–2825.
P. Bilalis, D. Katsigiannopoulos, A. Avgeropoulos, G. Sakellariou, Noncovalent functionalization of carbon nanotubes with polymers, RSC Adv., 6 (2014) 2911– 2934.
Y. K. Mishra, R. Adelung, ZnO tetrapod materials for functional applications, Mater. Today, 21 (2018) 631-651.
X. Wang, W. Tian, T. Zhai, C. Zhi, Y. Bando, D. Golberg, Cobalt (II, III) oxide hollow structures: fabrication, properties and applications, J. Mater. Chem., 22 (2012) 23310-23326.
N. S. Bobbitt, M. L. Mendonca, A. J. Howarth, T. Islamoglu, J. T. Hupp, O. K. Farha, R. Q. Snurr, Metal–organic frameworks for the removal of toxic industrial chemicals and chemical warfare agents, Chem. Soc. Rev., 46 (2017) 3357-3385.
D. Britt, D. Tranche Montagne, O. M. Yaghi, Metal–organic frameworks with high capacity and selectivity for harmful gases, Proc. Natl. Acad. Sci., 105 (2008) 11623–1162.
S. E. Lehman, S. C. Larsen, Zeolite and mesoporous silica nanomaterials: greener syntheses, environmental applications and biological toxicity, Environ. Sci. Nano, 1 (2014) 200-213.
N. Moitra, P. Trens, L. Raehm, J.O. Durand, X. Cattoen, M. Wong Chi Man, Facile route to functionalized mesoporous silica nanoparticles by click chemistry, J. Mater. Chem., 21 (2011) 13476-13482.
C. Yeom, Y. Kim, Mesoporous alumina with high capacity for carbon monoxide adsorption, Korean J. Chem. Eng., 35 (2017) 587–593.
A. Walcarius, L. Mercier, Mesoporous organosilicon adsorbents: Nano engineered materials for removal of organic and inorganic pollutants, J. Mater. Chem., 20 (2010) 4478–4511.
C. Yeom, R. Selvaraj, Y. Kim, Preparation of nonporous alumina using aluminum chloride via precipitation templating method for CO adsorbent, J. Ind. Eng. Chem., 67 (2017) 132-139.
Z. Li, J.C. Barnes, A. Bosoy, J.F. Stoddart, J.I. Zink, Mesoporous silica nanoparticles in biomedical applications, Chem. Soc. Rev., 41 (2012) 2590–2605.
T. Kitao, Y. Zhang, S. Kitagawa, B. Wang, T. Uemura, Hybridization of MOFs and polymers, Chem. Soc. Rev., 46 (2017) 3097–348.
X. Lian, Y. Fang, E. Joseph, Q. Wang, J. Li, S. Banerjee, C. Lollar, X. Wang, H. Zhou, Enzyme-MOF (metal-organic framework) composites, Chem. Soc. Rev., 46 (2017) 3386-3401.
L. Peng, J. Peng, Z. Xue, B. Han, J. Li, G. Yang, Large-pore mesoporous Mn3O4 crystals derived from metal-organic frameworks, Chem. Commun., 49 (2013) 11695-11697.
R. K. Bhakta, J. L. Herberg, B. Jacobs, A. Highley, R. B. Jr, N. W. Ockwig, J. A. Greathouse, M. Alendorf, Metal-organic frameworks as templates for nanoscale NaAlH4, J. Am. Chem. Soc., 131 (2009) 13198–13199.
S. Rengaraj, Y. Kim, J-W. Yeon, W-H. Kim, Application of Mg-mesoporous alumina prepared by using magnesium stearate as a template for the removal of nickel: kinetics, isotherm, and error analysis, Ind. Eng. Chem. Res., 46 (2007) 2834-2842.
L. Dejam, S. Solaymani, A. Achour, S. Stach, S. Talu, N. Beryani Nezafat, V. Dalouji, A.A. Shokri, A. Ghaderi, Correlation between surface topography, optical band gaps and crystalline properties of engineered AZO and CAZO thin films, Chem. Phys. Lett., 719 (2019) 78-90.
P. Souza Santos, H. Souza Santos, S.P. Toledo, Standard transition alumina. Electron microscopy studies, Mat. Res., 3 (2000) 104-114.
M. Macêdo, C. Bertran, C. Osawa, Kinetics of the γ → α -alumina phase transformation by quantitative X-ray diffraction, J. Mater. Sci., 42 (2007) 2830–2836.
C.H., Shek, J.K.L. Lai, T.S. Gu, G.M. Lin, Transformation evolution and infrared absorption spectra of amorphous and crystalline nano-Al2O3 powders, Nanostruct. Mater., 8 (1997) 605-610.
J.L. Peng, L.D. Lai, X. Jiang, W.J. Jiang, B. Lai, Catalytic ozonation of succinic acid in aqueous solution using the catalyst of Ni/Al2O3 prepared by electroless plating-calcination method, Sep. Purif. Technol,. 195(2018) 138–148.
N. Mozaffari, N. Mozaffari, S. M. Elahi, S. Vambol, V. Vambol, N. A. Khan, N. Khan, Kinetics study of CO molecules adsorption on Al2O3/Zeolite composite films prepared by roll-coating method, Surface Engineering. (2020) 1-10. https://doi.org/10.1080/02670844.2020.1768628
M. Trueba, S.P. Trasatti, γ-Alumina as a support for catalysts: a review of fundamental aspects, Eur. J. Inorg. Chem., 17 (2005) 3393-3403.
G. Busca, The surface of transitional aluminas: a critical review, Catal. Today, 226 (2014) 2-13.
I. Levin, D. Brandon, Metastable alumina polymorphs: crystal structure and transition sequences, J. Am. Ceramic Soc., 81 (1998) 1995-2012.
N. Mozaffari, S. Solaymani, A. Achour, S. Kulesza, M. Bramowicz, N. B. Nezafat, Ş. Ţălu, N. Mozaffari, S. Rezaee, New insights into SnO2/Al2O3, Ni/Al2O3, and SnO2/Ni/Al2O3compositefilms for CO adsorption: building a bridge between microstructures and adsorption properties, J. Phys. Chem. C, 124 (2020) 3692–3701.
I. W. Davies, L. Matty, D. L. Hughes and P. J. Reider, Are heterogeneous catalysts precursors to homogeneous catalysts, Chem. Soc., 123 (2001) 10139-10140.
I. Saldan, Y. Semenyuk, I. Marchuk, O. Reshetnyak, Chemical synthesis and application of palladium nanoparticles, J. Mater. Sci., 50 (2015) 2337-2354.
N. Ono, The Nitro Group in Organic Synthesis; Wiley-VCH: Weinheim, 392, (2001). Doi: 10.1021/op010046s.
G. Yan, M. Yang, Recent advances in the synthesis of aromatic nitro compounds. Org. Biomol. Chem., 11 (2013) 2554-2566.
P. LaBeaume, M. Placzek, M. Daniels, I. Kendrick, P. Ng, M. McNeel, R. Afroze, A. Alexander, R. Thomas, A. E. Kallmerten, G. B. Jones, Microwave-accelerated fluorodenitrations and nitrodehalogenations: expeditious routes to labeled PET ligands and fluoropharmaceuticals, Tetrahedron Lett., 51 (2010) 1906-1909.
X.-F. Wu, J. Schranck, H. Neumann, M. Beller, Convenient and mild synthesis of nitroarenes by metal-free nitration of arylboronic acids, Chem. Commun., 47 (2011) 12462-12463.
J. P. Das, P. Sinha, S. Roy, A Nitro-Hunsdiecker Reaction: From Unsaturated Carboxylic Acids to Nitrostyrenes and Nitroarenes, Org. Lett., 4 (2002) 3055-3058.
J. J. Song, J. T. Reeves, F. Gallou, Z. Tan, N. K. Yee, C. H Senanayake, Organometallic methods for the synthesis and functionalization of azaindoles, Chem. Soc. Rev., 36 (2007) 1120-1132.
S. Mohan, P. Dinesha, S. Kumar, NOx reduction behaviour in copper zeolite catalysts for ammonia SCR systems: A review, Chem. Eng. J., 384 (2019) 123253.
L. Tosheva, V.P. Valtchev, Nanozeolites: synthesis, crystallization mechanism, and applications, Chem. Mater., 17 (2005) 2494-2513.
G. T. Vuong, V. T. Hoang, D. T. Nguyen, T. O. Do, Synthesis of nanozeolites and nanozeolite-based FCC catalysts, and their catalytic activity in gas oil cracking reaction, Appl. Catal. A, 382 (2010) 231-239.
H. Konno, T. Okamura, T. Kawahara, Y. Nakasaka, T. Tago, T. Masuda, Kinetics of n-hexane cracking over ZSM-5 zeolites – Effect of crystal size on effectiveness factor and catalyst lifetime, Chem. Eng. J., 207–208 (2012) 490-496.
T. Tago, H. Konno, Y. Nakasaka, T. Masuda, Size-controlled synthesis of nano-zeolites and their application to light olefin synthesis, Catal. Surv. Asia, 16 (2012) 148-163.
T. Tago, H. Konno, M. Sakamoto, Y. Nakasaka, T. Masuda, Selective synthesis for light olefins from acetone over ZSM-5 zeolites with nano-and macro-crystal sizes, Appl. Catal., A, 403 (2011) 183-191.
K. Na, C. Jo, J. Kim, K. Cho, J. Jung, Y. Seo, R.J. Messinger, B.F. Chmelka, R. Ryoo, Directing Zeolite Structures into Hierarchically Nanoporous Architectures, Sci., 333 (2011) 328-332.
J. He, D. Chen, N. Li, Q. Xu, H. Li, J. He, J. Lu, Controlled fabrication of mesoporous ZSM-5 Zeolite-supported PdCu alloy nanoparticles for complete oxidation of toluene, Appl. Catal. B: Environ., 265 (2019) 118560. http://doi: 10.1016/j.apcatb.2019.118560.
S. van Donk, A.H. Janssen, J.H. Bitter, K.P. de Jong, Generation, characterization, and impact of mesopores in zeolite catalysts, Catal. Rev., 45 (2003) 297-319.
R. Chun, S. Kim, S. H. Han, A. K. Pandey, N. K. Mishra, I. S. Kim, Site-selective C−H nitration of N-aryl-7-azaindoles under palladium(II) catalysis, Tetrahedron Lett., 59 (2018) 3848-3852.
S. Zhang, T. Shao, The impacts of aggregation and surface chemistry of carbon nanotubes on the adsorption of synthetic organic compounds, Environ. Sci. Technol., 43 (2009) 5719-5725.
V. Sabna, S.G. Thampi, S. Chandrakaran, Adsorption of crystal violet onto functionalised multi-walled carbon nanotubes: Equilibrium and kinetic studies, Ecotoxicol. Environ. Saf., 134 (2016) 390–397.
H. Anjum, K. Johari, N. Gnanasundaram, M. Ganesapillai, A. Arunagiri, I. Regupathi, M. Thanabalan, A review on adsorptive removal of oil pollutants (BTEX) from wastewater using carbon nanotubes, J. Mol. Liq., 277 (2019) 1005–1025.
S. Mallakpour, S. Rashidimoghadam, 9 - Carbon Nanotubes for Dyes Removal, Composite Nanoadsorbents, Micro Nano Technol., Elsevier, (2019) 211-243. http://doi: 10.1016/B978-0-12-814132-8.00010-1.
D.P. Dutta, R. Venugopalan, S. Chopade, Manipulating carbon nanotubes for efficient removal of both cationic and anionic dyes from wastewater, Chem. Select., 2 (2017) 3878–3888.
N, Mozaffari. A, H, S, Mirzahosseini. A, H, Sari. L, F, Aval. Investigation of carbon monoxide gas adsorption on the Al2O3/Pd(NO3)2/zeolite composite film. Journal of Theoretical Appl. Phys., 14 (2019) 65–74.
L. Samain, A. Jaworski, M. Edén, D. M. Ladd, D. K. Seo, F. J. Garcia-Garciad, U. Häussermanna, structural analysis of highly porous γ-Al2O3, J. Solid State Chem., 217 (2014)1–8.
M. Shayesteh, M. S. Afarani, A. Samimi1, M. Khorram, Preparation of γ-Al2O3 and prioritization of affecting factors on the crystallite size using taguchi method, Trans. Phenom. Nano Micro Scales, 1 (2013) 45-52.
J. Liu, j. Zhang, H. Zhang, F. Zhang, M. Zhu, N. Hu, X. Chen, H. Kita, Synthesis of hierarchical zeolite T nanocrystals with the assistance of zeolite seed solution, J. Solid State Chem., 285 (2020) 121228.
N. M. Mahmoodi, M. H. Saffar-Dastgerdi, Zeolite nanoparticle as a superior adsorbent with high capacity: Synthesis, surface modification and pollutant adsorption ability from wastewater, Microchem. J., 145 (2019) 74-83.
J.H. Kwak, J. Hu, D. Mei, C.W. Yi, D.H. Kim, C.H. Peden, L.F. Allard, J. Szanyi, Coordinatively unsaturated Al3+ centers as binding sites for active catalyst phases of platinum on gamma-Al2O3, Sci., 325 (2009) 1670-1673.
S. Arnis, F. Belaib, M. Bencheikh Lehocine, H.-A Menian, Progress in clean energy, analysis and modeling,, Equilbrium and kinetic studies of adsorption of Cd(II), Zn(II), and Cu(II) from aqueous solution into cereal by-products, chapter 4, volume 1, Springer Publishing, (2015). https://doi: 10.1007/978-3-319-16709-1
D.M. Ruthven, principle of adsorption and desorption process, Wiley, New York, (1984).
S.H. Chien, W.R. Clayton, Application of elovich equation to the kinetics of phosphate release and sorption in soils, Soli Sci. Soc. Am. j., 44 (1980) 265-268.
A.O. Dada, D. F. Latona, O. J. Ojediran, O. O. Nath, Adsorption of Cu (II) onto bamboo supported manganese (BS-Mn) nanocomposite: effect of operational parameters, kinetic, isotherms, and thermodynamic studies, J. Appl. Sci. Environ. Manage., 20 (2016) 409 –422.
S. Karka, S. Kodukula, S. V. Nandury, U. Pal, Polyethylenimine-modified zeolite 13X for CO2 capture: adsorption and kinetic studies, ACS Omega, 4(2019) 16441 – 16449.
A.A. Inyinbor, F.A. Adekola, G.A. Olatunji, Kinetics, isotherms and thermodynamic modeling of liquid phase adsorption of Rhodamine B dye onto Raphia hookerie fruit epicarp, Water Res. Ind., 15 (2016) 14–27.
Y.S. Ho, G. McKay, Application of kinetic models to the sorption of copper (II) on to peat, Adsorp. Sci. Technol., 20 (2002) 797-815.
Copyright (c) 2020 Analytical Methods in Environmental Chemistry Journal

This work is licensed under a Creative Commons Attribution 4.0 International License.
JOURNAL PUBLISHING AGREEMENT
PLEASE PROVIDE US THE FOLLOWING INFORMATION,
Article entitled:
Corresponding author:
To be published in the journal:
Your Status
I am the sole author of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
I am one author signing on behalf of all co-authors of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
Please tick the above blanks (as appropriate), review the Journal Publishing Agreement, and then sign and date the document in black ink.
Published Journal Article: the author may share a link to the formal publication through the relevant DOI. Additionally theses and dissertations which contain embedded Published Journal Articles as part of the formal submission may be hosted publicly by the awarding institution with a link to the formal publication through the relevant DOI. Any other sharing of Published Journal Articles is by agreement with the publisher only.
Signed: ______________________________________ Name printed: ___________________________________________
Title and Company (if employer representative): _______________________Date: __________________________________