A new analytical method based on Co-Mo nanoparticles supported by carbon nanotubes for removal of mercury vapor from the air by the amalgamation of solid-phase air removal
Volume 5, Issue 01, Pages 22-35, Mar 2022 *** Field: Analytical method in occupational health
Abstract
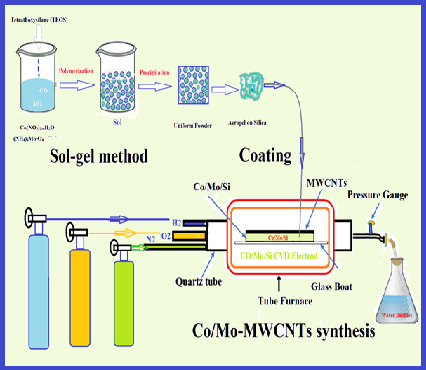
Heavy metals are a major cause of environmental pollution, and mercury is a well-known toxicant that is extremely harmful to the environment and human health. In this study, new carbon nanotubes coated with cobalt and molybdenum nanoparticles (Co-Mo/MWCNT) were used for Hg0 removal from the air by the amalgamation of solid-phase air removal method (ASPAR). In the bench-scale setup, the mercury vapor in air composition was produced by the mercury vapor generation system (HgGS) and restored in a polyethylene airbag . In optimized conditions, the mercury vapor in the airbag passed through Co-Mo/MWCNT and was absorbed on it. Then, the mercury was completely desorbed from Co-Mo/MWCNT by increasing temperature up to 220 °C and online determined by cold vapor atomic absorption spectrometry (CV-AAS). The recovery and capacity of Co-Mo/MWCNT were obtained at 98% and 191.3 mg g-1, respectively. The Repeatability of the method was 32 times. The mercury vapors absorbed on Co-Mo/MWCNT adsorbent could be maintained at 7 days at the refrigerator temperature. The Co-Mo/MWCNT as a sorbent has many advantages such as; high capacity, renewable, good repeatability and chemical adsorption (amalgamation) of mercury removal from the air. The method was successfully validated by MCA and spiking of real samples.
References
H. Wang, S. Wang, Y. Duan, Y.-n. Li, Y. Xue, Z. Ying, Activated carbon for capturing Hg in flue gas under O2/CO2 combustion conditions. Part 1: Experimental and kinetic study, Energy Fuels, 32 (2018) 1900-1906. https://doi.org/10.1021/acs.energyfuels.7b03380.
X. Wu, Y. Duan, N. Li, P. Hu, T. Yao, J. Meng, S. Ren, H. Wei, Regenerable Ce–Mn/TiO2 catalytic sorbent for mercury removal with high resistance to SO2, Energy Fuels, 33 (2019) 8835-8842. https://doi.org/10.1021/acs.energyfuels.9b00978.
G. Genchi, M.S. Sinicropi, A. Carocci, G. Lauria, A. Catalano, Mercury exposure and heart diseases, Int. J. Environ. Res. Public. Health, 14 (2017) 74. https://doi.org/10.3390/ijerph14010074.
S.E. Orr, C.C. Bridges, Chronic kidney disease and exposure to nephrotoxic metals, Int. J. Mol. Sci., 18 (2017) 1039. https://doi.org/10.3390/ijms18051039.
E.G. Pacyna, J.M. Pacyna, F. Steenhuisen, S. Wilson, Global anthropogenic mercury emission inventory for 2000, Atmos. Environ., 40 (2006) 4048-4063. https://doi.org/10.1016/j.atmosenv.2006.03.041.
C. Winder, N.H. Stacey, Occupational toxicology, 2 ed, CRC press, 2004. https://www.routledge.com/Occupational-Toxicology/Winder-Stacey/p/book/9780367394554
M. Sakamoto, N. Tatsuta, K. Izumo, P.T. Phan, L.D. Vu, M. Yamamoto, M. Nakamura, K. Nakai, K. Murata, Health impacts and biomarkers of prenatal exposure to methylmercury: Lessons from Minamata, Japan, Toxins, 6 (2018). https://doi.org/10.3390/toxics6030045.
UNEP, Minamata convention on mercury, United Nations environment programme Geneva, Switzerland, 2013. https://www.unep.org/resources/report/minamata-convention-mercury.
R. Andrews, P.F. O’Connor, NIOSH manual of analytical methods (NMAM), 2020. https://www.cdc.gov/niosh/nmam/default.html.
A. Ebrahimi, A. Salarifar, Air pollution Analysis: Nickel paste on Multi-walled carbon nanotubes as a novel adsorbent for the mercury removal from air, Anal. methods. Environ. chem. J., 2 (2019) 79-88. https://doi.org/10.24200/amecj.v2.i03.70.
Y. Liu, Y. Li, H. Xu, J. Xu, Oxidation removal of gaseous Hg0 using enhanced-Fenton system in a bubble column reactor, Fuel, 246 (2019) 358-364. https://doi.org/10.1016/j.fuel.2019.03.018.
S. Zhang, M. Díaz-Somoano, Y. Zhao, J. Yang, J. Zhang, Research on the mechanism of elemental mercury removal over Mn-Based SCR catalysts by a developed Hg-TPD method, Energy Fuels, 33 (2019) 2467-2476. https://doi.org/10.1021/acs.energyfuels.8b04424.
H. Zeng, F. Jin, J. Guo, Removal of elemental mercury from coal combustion flue gas by chloride-impregnated activated carbon, Fuel, 83 (2004) 143-146. https://doi.org/10.1016/S0016-2361(03)00235-7.
B. Zhang, P. Xu, Y. Qiu, Q. Yu, J. Ma, H. Wu, G. Luo, M. Xu, H. Yao, Increasing oxygen functional groups of activated carbon with non-thermal plasma to enhance mercury removal efficiency for flue gases, Chem. Eng. J., 263 (2015) 1-8. https://doi.org/10.1016/j.cej.2014.10.090.
H. Li, S. Wu, C.Y. Wu, J. Wang, L. Li, K. Shih, SCR atmosphere induced reduction of oxidized mercury over CuO-CeO2/TiO2 catalyst, Environ. Sci. Technol., 49 (2015) 7373-7379. https://doi.org/10.1021/acs.est.5b01104.
J. Wu, C. Li, X. Zhao, Q. Wu, X. Qi, X. Chen, T. Hu, Y. Cao, Photocatalytic oxidation of gas-phase Hg0 by CuO/TiO2, App. Catal. B, 176-177 (2015) 559-569. https://doi.org/10.1016/j.apcatb.2015.04.044.
Y. Liu, J. Zhang, J. Pan, Photochemical oxidation removal of Hg0 from flue gas Containing SO2/NO by an ultraviolet irradiation/hydrogen peroxide (UV/H2O2) process, Energy Fuels, 28 (2014) 2135-2143. https://doi.org/10.1021/ef401697y.
Y. Liu, Y. Wang, Gaseous elemental mercury removal using VUV and heat coactivation of Oxone/H2O/O2 in a VUV-spraying reactor, Fuel, 243 (2019) 352-361. https://doi.org/10.1016/j.fuel.2019.01.130.
D. Liu, W. Zhou, J. Wu, Kinetic behavior of elemental mercury sorption on cerium- and lanthanum-based composite oxides, Surf. Rev. Lett., 26 (2019) 1-9. http://dx.doi.org/10.1142/S0218625X1850141X.
G. Li, Q. Wu, S. Wang, Z. Li, H. Liang, Y. Tang, M. Zhao, L. Chen, K. Liu, F. Wang, The influence of flue gas components and activated carbon injection on mercury capture of municipal solid waste incineration in China, Chem. Eng. J., 326 (2017) 561-569. https://doi.org/10.1016/j.cej.2017.05.099.
H. Yang, Z. Xu, M. Fan, A.E. Bland, R.R. Judkins, Adsorbents for capturing mercury in coal-fired boiler flue gas, J. Hazard. Mater., 146 (2007) 1-11. https://doi.org/10.1016/j.jhazmat.2007.04.113.
Z. Tan, L. Sun, J. Xiang, H. Zeng, Z. Liu, S. Hu, J. Qiu, Gas-phase elemental mercury removal by novel carbon-based sorbents, Carbon, 50 (2012) 362-371. https://doi.org/10.1016/j.carbon.2011.08.036.
Y. Zheng, A.D. Jensen, C. Windelin, F. Jensen, Dynamic measurement of mercury adsorption and oxidation on activated carbon in simulated cement kiln flue gas, Fuel, 93 (2012) 649-657. https://doi.org/10.1016/j.fuel.2011.09.053.
W. Xu, Y.G. Adewuyi, Y. Liu, Y. Wang, Removal of elemental mercury from flue gas using CuOx and CeO2 modified rice straw chars enhanced by ultrasound, Fuel Proc. Technol., 170 (2018) 21-31. https://doi.org/10.1016/j.fuproc.2017.10.017.
H. Xu, Z. Qu, C. Zong, W. Huang, F. Quan, N. Yan, MnOx/graphene for the catalytic oxidation and adsorption of elemental mercury, Environ. Sci. Technol., 49 (2015) 6823-6830. https://doi.org/10.1021/es505978n.
Y. Ma, B. Mu, X. Zhang, H. Xu, Z. Qu, L. Gao, B. Li, J. Tian, Ag-Fe3O4@rGO ternary magnetic adsorbent for gaseous elemental mercury removal from coal-fired flue gas, Fuel, 239 (2019) 579-586. https://doi.org/10.1016/j.fuel.2018.11.065.
B. Zhao, X. Liu, Z. Zhou, H. Shao, M. Xu, Catalytic oxidation of elemental mercury by Mn–Mo/CNT at low temperature, Chem. Eng. J., 284 (2016) 1233-1241. https://doi.org/10.1016/j.cej.2015.09.090.
J. Yang, Y. Zhao, S. Liang, S. Zhang, S. Ma, H. Li, J. Zhang, C. Zheng, Magnetic iron–manganese binary oxide supported on carbon nanofiber (Fe3−xMnxO4/CNF) for efficient removal of Hg0 from coal combustion flue gas, Chem. Eng. J., 334 (2018) 216-224. https://doi.org/10.1016/j.cej.2017.10.004.
X. Zhang, B. Shen, S. Zhu, H. Xu, L. Tian, UiO-66 and its Br-modified derivates for elemental mercury removal, J. Hazard. Mater., 320 (2016) 556-563. https://doi.org/10.1016/j.jhazmat.2016.08.039.
B. Tawabini, A. A, M. Khaled, Removal of mercury from water by multi-walled carbon nanotubes, Water Sci. Technol., 61 (2010) 591-598. https://doi.org/10.2166/wst.2010.897.
F. Golbabaei, A. Ebrahimi, H. Shirkhanloo, A. Koohpaei, A. Faghihi-Zarandi, Single-walled carbon nanotubes (SWCNTs), as a novel sorbent for determination of mercury in air, Glob. J. Health Sci., 8 (2015) 273. https://doi.org/10.5539/gjhs.v8n7p273.
Y. Ma, D. Zhang, H. Sun, J. Wu, P. Liang, H. Zhang, Fe–Ce mixed oxides supported on carbon nanotubes for simultaneous removal of NO and Hg0 in flue gas, Ind. Eng. Chem. Res., 57 (2018) 3187-3194. https://doi.org/10.1021/acs.iecr.8b00015.
W. Xu, A. Hussain, Y. Liu, A review on modification methods of adsorbents for elemental mercury from flue gas, Chem. Eng. J., 346 (2018) 692-711. https://doi.org/10.1016/j.cej.2018.03.049.
Z. Shen, J. Ma, Z. Mei, J. Zhang, Metal chlorides loaded on activated carbon to capture elemental mercury, J. Environ. Sci., 22 (2010) 1814-1819. https://doi.org/10.1016/S1001-0742(09)60324-7.
Y. Liu, Y. Wang, H. Wang, Z. Wu, Catalytic oxidation of gas-phase mercury over Co/TiO2 catalysts prepared by sol–gel method, Catal. Commun., 12 (2011) 1291-1294. http://dx.doi.org/10.1016%2Fj.catcom.2011.04.017.
S. Zhao, Z. Li, Z. Qu, N. Yan, W. Huang, W. Chen, H. Xu, Co-benefit of Ag and Mo for the catalytic oxidation of elemental mercury, Fuel, 158 (2015) 891-897. https://doi.org/10.1016/j.fuel.2015.05.034.
L.C. Klein, Sol-Gel Processing of Silicates, Annu. Rev. Mater. Sci., 15 (1985) 227-248. https://doi.org/10.1146/annurev.ms.15.080185.001303.
L.M. Hoyos-Palacio, A.G. García, J. F. Pérez-Robles, J. González, H.V. Martínez-Tejada, Catalytic effect of Fe, Ni, Co and Mo on the CNTs production, IOP, Con. Ser. Mater. Sci. Eng., 59 (2014) 012005. https://doi.org/10.1088/1757-899X/59/1/012005.
V.M. Irurzun, Y. Tan, D.E. Resasco, Sol−Gel synthesis and characterization of Co−Mo/Silica catalysts for single-walled carbon nanotube production, Chem. Mater., 21 (2009) 2238-2246. https://doi.org/10.1021/cm900250k.
G. Luo, H. Yao, M. Xu, X. Cui, W. Chen, R. Gupta, Z. Xu, Carbon nanotube-silver composite for mercury capture and analysis, Energy Fuels, 24 (2010) 419-426. https://doi.org/10.1021/ef900777v.
H. Shirkhanloo, M. Osanloo, M. Ghazaghi, H. Hassani, Validation of a new and cost-effective method for mercury vapor removal based on silver nanoparticles coating on micro glassy balls, Atmos. Pollut. Res., 8 (2017) 359-365. https://doi.org/10.1016/j.apr.2016.10.004.
H. Shirkhanloo, F. Golbabaei, A. Vahid, A. Faghihi Zarandi, A novel nano-palladium embedded on the mesoporous silica nanoparticles for mercury vapor removal from air by the gas field separation consolidation process, Appl. Nanosci., (2022). https://doi.org/10.1007/s13204-022-02366-0.
Y. Ma, T. Xu, X. Zhang, Z. Fei, H. Zhang, H. Xu, Y. Ma, Manganese bridge of mercury and oxygen for elemental mercury capture from industrial flue gas in layered Mn/MCM-22 zeolite, Fuel, 283 (2021) 118973. https://doi.org/10.1016/j.fuel.2020.118973.
H. Zhang, J. Wang, T. Liu, M. Zhang, L. Hao, T. Phoutthavong, P. Liang, Cu-Zn oxides nanoparticles supported on SBA-15 zeolite as a novel adsorbent for simultaneous removal of H2S and Hg0 in natural gas, Chem. Eng. J., 426 (2021) 131286. https://doi.org/10.1016/j.cej.2021.131286.
JOURNAL PUBLISHING AGREEMENT
PLEASE PROVIDE US THE FOLLOWING INFORMATION,
Article entitled:
Corresponding author:
To be published in the journal:
Your Status
I am the sole author of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
I am one author signing on behalf of all co-authors of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
Please tick the above blanks (as appropriate), review the Journal Publishing Agreement, and then sign and date the document in black ink.
Published Journal Article: the author may share a link to the formal publication through the relevant DOI. Additionally theses and dissertations which contain embedded Published Journal Articles as part of the formal submission may be hosted publicly by the awarding institution with a link to the formal publication through the relevant DOI. Any other sharing of Published Journal Articles is by agreement with the publisher only.
Signed: ______________________________________ Name printed: ___________________________________________
Title and Company (if employer representative): _______________________Date: __________________________________