Analysis of complexation between new bidentate bis-NHC ligand and some metal cations at different temperature
Volume 5, Issue 02, Pages 5-23, Jun 2022 *** Field:Analysis of Environment samples
Abstract
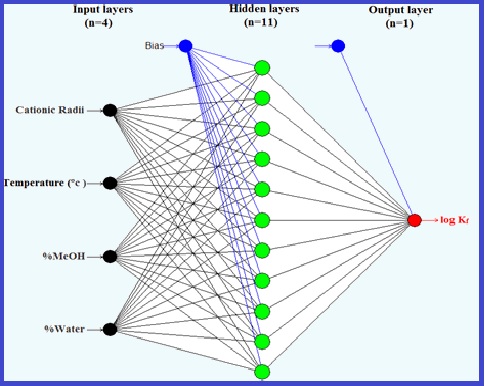
In this research, the determination and complexation process between 3,3'-(2,2'-(4-methyl-phenylenesulfonamido)bis(ethane-2,1-diyl))bis(1-benzyl-3H-benzo[d]imidazol-1-ium)dibromide with Ni2+, Zn2+, Pd2+, Ag+, and Hg2+ cations in the binary mixture of methanol (MeOH) and water (H2O) at different temperatures (15, 25, 35 and 45ºC) were studied using a conductometric method. The results show that the stoichiometry of the complexes in all binary mixed solvents for Ni2+, Zn2+, and Pd2+ were 1:1 (M:L), while in other cases 1:2 (M:L) and 2:1(M:L). The stability constants (log ) of complex formation have been determined by fitting molar conductivity curves using a computer program (GENPLOT). The obtained data shows that in the pure methanol solvent system, the stability order is Ni2+< Pd2+<Zn2+<Hg2+<Ag+ and the complexation process seems more stable in pure methanol in most cases. The thermodynamic parameters were determined conductometrically. The complexes in all cases were found to be enthalpy destabilized but entropy stabilized. The experimental data was tested by using an artificial neural network (ANN) program and was in good agreement with the estimated data.
References
H.W. Wanzlick, H.J. Schönherr, Direct synthesis of a mercury salt‐carbene complex, Angewandte Chemie International Edition in English, 7 (1968) 141-142. https://doi.org/10.1002/anie.196801412
A.J. Arduengo, R.L. Harlow, M. Kline, A stable crystalline carbene, J. Am. Chem.l Soc., 113 (1991) 361-363. https://doi.org/10.1021/ja00001a054
R.A. Haque, A. Washeel Salman, New N-heterocyclic carbene mercury(II) complexes: Close mercury–arene interaction, J. Organometal. Chem., 696 (2011) 3507-3512. https://doi.org/10.1016/j.jorganchem.2011.07.032
C.M. Crudden, D.P. Allen, Stability and reactivity of N-heterocyclic carbene complexes, Coor. Chem. Rev., 248 (2004) 2247-2273. https://doi.org/10.1016/j.ccr.2004.05.013
S. Budagumpi, R.A. Haque, A.W. Salman, Stereochemical and structural characteristics of single- and double-site Pd(II)–N-heterocyclic carbene complexes: Promising catalysts in organic syntheses ranging from CC coupling to olefin polymerizations,. Coord. Chem. Rev., 256 (2012) 1787-1830. https://doi.org/10.1016/j.ccr.2012.04.003
M. Rezayi, Y.H. Lee, M.M. Abdi, N.M.D. Noran, C. Esmaeili, S. B. Tavakoly Sany, A. Salleh, L. Narimani, N. Saadati, F. Tajalli, A thermodynamic study on the complex formation between tris (2-Pyridyl) methylamine (tpm) with Fe II, Fe III, Cu II and Cr III cations in water, acetonitrile binary solutions using the conductometric method, Int. J. Electrochem. Sci., 8 (2013) 6922-6932. http://www.electrochemsci.org/
M. Y. Khorasani, H. Langari, S.B. Tavakoly Sany, M. Rezayi, A. Sahebkar, The role of curcumin and its derivatives in sensory applications, Mater. Sci. Eng. C, 103 (2019) 109792. https://doi.org/ 10.1016/j.msec.2019.109792
S.B. Tavakoly Sany, R. Hashim, A. Salleh, M. Rezayi, O. Safari, Ecological quality assessment based on macrobenthic assemblages indices along West Port, Malaysia coast. Environ. Earth Sci., 74 (2015) 1331-1341. https://doi.org/10.1007/s12665-015-4122-3
S.B. Tavakoly Sany, R. Hashim, M. Rezayi, M.A.Rahman, B.B.M. Razavizadeh, E. Abouzari-lotf, D.J. Karlen, Integrated ecological risk assessment of dioxin compounds. Environ. Sci. Pollut. Res., 22 (2015) 11193-11208. https://doi.org/ 10.1007/s11356-015-4511-x
J. Houghton, G. Dyson, R.E. Douthwaite,A.C. Whitwood, B. Kariuki, Structural variation, dynamics, and catalytic application of palladium(II) complexes of di-N-heterocyclic carbene-amine ligands, Dalton Trans, 28 (2007) 3065-73. https://doi.org/10.1039/b703248j
C.Y Liao, K.T. Chan, J.Y. Zeng, C.H. Hu, C.Y. Tu, H.M. Lee., Nonchelate and chelate complexes of Palladium (II) with N-heterocyclic carbene ligands of amido functionality, Organometallics, 26 (2007) 1692-1702. https://doi.org/10.1021/om0610041
Q.X. Liu, L.X. Zhao, X.J. Zhao, Z.X. Zhao, Z.Q. Wang, A.H. Chen, X.G. Wang, Silver (I), palladium (II) and mercury (II) NHC complexes based on bis-benzimidazole salts with mesitylene linker: Synthesis, structural studies and catalytic activity, J. Organomet. Chem., 731(2013) 35-48. https://doi.org/10.1016/j.jorganchem.2013.01.026
S.B. Tavakoly Sany, R. Hashim, A. Salleh, O. Safari, A. Mehdinia, M. Rezayi, Risk assessment of polycyclic aromatic hydrocarbons in the West Port semi-enclosed basin (Malaysia), Environ. Earth Sci., 71 (2014) 4319-4332. https://doi.org/10.1007/s12665-013-2826-9
R.A. Haque, M.A. Iqbal, M.B Khadeer Ahamed, A.M.S. Abdul Majid, Z.A Abdul Hameed. Design, synthesis and structural studies of meta-xylyl linked bis-benzimidazolium salts: Potential anticancer agents against'human colon cancer, Chem. Cent. J., 6 (2012) 68. https://doi.org/ 10.1186/1752-153X-6-68
K.A. Abu-Safieh, A.S. Abu-Surrah, H.D. Tabba, H.A. AlMasri, R.M. Bawadi, F.M. Boudjelal, L.H. Tahtamouni, Novel palladium(II) and platinum(II) complexes with 1H-benzimidazol-2-ylmethyl-N-(4-bromo-phenyl)-amine: structural studies and anticancer activity, Eur. J. Med. Chem., 47 (2012) 399-411. https://doi.org/10.1016/j.ejmech.2011.11.008
R.A. Haque, M.Z. Ghdhayeb, S. Budagumpi, A.W. Salman, M.B. Khadeer Ahamed, A.M.S Abdul Majid, Non-symmetrically substituted N-heterocyclic carbene–Ag(I) complexes of benzimidazol-2-ylidenes: Synthesis, crystal structures, anticancer activity and transmetallation studies, Inorg. Chim. Acta, 394 (2013) 519-525. https://doi.org/10.1016/j.ica.2012.09.013
M. Darroudi, M. Gholami, M. Rezayi, M. Khazaei, An overview and bibliometric analysis on the colorectal cancer therapy by magnetic functionalized nanoparticles for the responsive and targeted drug delivery, J. Nanobiotechnol., 19 (2021) 1-20. https://doi.org/10.1186/s12951-021-01150-6
B. Hatamluyi, R. Sadeghian, S.B. Tavakoly Sany, I. Alipourfard, M. Rezayi, Dual-signaling electrochemical ratiometric strategy for simultaneous quantification of anticancer drugs, Talanta, 234 (2021) 122662. https://doi.org/10.1016/j.talanta.2021.122662
N. N. Al-Mohammed, Y. Alias, Z. Abdullah, R.M. Shakir, E.M. Taha, A. Abdul Hamid, Synthesis and antibacterial evaluation of some novel imidazole and benzimidazole sulfonamides, Molecules, 18 (2013) 11978-95. https://doi.org/10.3390/molecules181011978.
R.E. Douthwaite, J. Houghton, B.M. Kariuki, The synthesis of a di-N-heterocyclic carbene-amido complex of palladium(II), Chem. Commun. (Camb), 10 (2004) 698-9. https://doi.org/10.1039/b314814a
D.J. Nielsen, K.J. Cavell, B. Skelton, A. White, A pyridine bridged dicarbene ligand and its silver (I) and palladium (II) complexes: synthesis, structures, and catalytic applications, Inorg. Chim. Acta, 327(2002) 116-125. https://doi.org/10.1016/S0020-1693(01)00677-6
F.J.B Dit Dominique, H. Gornitzka, A Sournia-Saquet, C. Hemmert, Dinuclear gold(I) and gold(III) complexes of bridging functionalized bis(N-heterocyclic carbene) ligands: synthesis, structural, spectroscopic and electrochemical characterizations. Dalton Trans, (2009) 340-52. https://doi.org/10.1039/b809943j
U.J. Scheele, S. Dechert, F. Meyer, Bridged dinucleating N-heterocyclic carbene ligands and their double helical mercury(II) complexes, Inorg. Chim. Acta, 359 (2006) 4891-4900. https://doi.org/10.1016/j.ica.2006.08.048
B.A. Shah, F.A. Christy, P.S. Shrivastav, M. SanyalShah, Study on complex formation of dicyclohexyl-18-crown-6 with Mg2+, Ca2+ and Sr2+ in acetonitrile-water binary mixtures by conductometry, J. Phys. Chem. Sci., 1 (2014). https://doi.org/10.15297/JPCS.V1I2.02
M. Joshaghani, M.B. Gholivand, F. Ahmadi, Spectrophotometric and conductometric study of complexation of salophen and some transition metal ions in nonaqueous polar solvents, Spectrochim. Acta A, Mol. Biomol. Spect., 70 (2008) 1073-1078. https://doi.org/10.1016/j.saa.2007.10.015
A. Nezhadali, G.Taslimi, Thermodynamic study of complex formation between 3,5-di iodo-hydroxy quinoline and Zn2+, Ni2+ and Co2+ cations in some binary solvents using a conductometric method, Alexandria Eng. J., 52 (2013) 797-800. htpps://doi.org/ 10.1016/j.aej.2013.08.007
M. Rezayi, S. Ahmadzadeh, A. Kassim, L.Y. Heng, Thermodynamic studies of complex formation between Co (Salen) ionophore with chromate (II) ions in AN-H2O binary solutions by the conductometric method, Int. J. Electrochem. Sci, 6 (2011) 6350-6359. http://www.electrochemsci.org/
E. R. Enemo, T.E. Ezenwa, E.C. Nleonu, Determination of formation constants and thermodynamic Parameters of chromium (III) ions with some ligands by conductometry, IOSR J. Appl. Chem., 12 (2019) 54-58. htpps://doi.org/ 10.9790/5736-1210015458
M. Rezayi ,Y. Alias, M.M. Abdi, K. Saeedfar, N. Saadati, Conductance studies on complex formation between c-methylcalix[4]resorcinarene and titanium (III) in acetonitrile-H(2)O binary solutions, Molecules, 18 (2013) 12041-12050. https://doi.org/10.3390/molecules181012041
L. Rao, G. Tian, Thermodynamic study of the complexation of uranium(VI) with nitrate at variable temperatures,. J. Chem. Thermodyn., 40 (2008) 1001-1006. https://doi.org/10.1016/j.jct.2008.02.013
S. Jaubert, G. Maurer, Quantitative NMR spectroscopy of binary liquid mixtures (aldehyde+alcohol) Part I: Acetaldehyde+(methanol or ethanol or 1-propanol), J. Chem. Thermodyn., 68 (2014) 332-342. https://doi.org/10.1016/j.jct.2013.03.022
B. Ghalami-Choobar, N. Mahmoodi, P. Mossayyebzadeh-Shalkoohi, Thermodynamic properties determination of ternary mixture (NaCl+Na2HPO4+water) using potentiometric measurements, J. Chem. Thermodyn., 57 (2013) 108-113. https://doi.org/10.1016/j.jct.2012.08.011
M. Rezayi, L.Y. Heng, A. Kassim, S. Ahmadzadeh, Y. Abdollahi, H. Jahangirian, Immobilization of Ionophore and Surface Characterization Studies of the Titanium (III) Ion in a PVC-Membrane Sensor, Sensors, 12 (2012) 8806-8814. https://doi.org/10.3390/s120708806
M. Rezayi, L.Y. Heng, A. Kassim, S. Ahmadzadeh, Y. Abdollahi, H. Jahangirian, Immobilization of tris (2 pyridyl) methylamine in PVC-membrane sensor and characterization of the membrane properties, Chem. Cent. J., 6 (2012) 40. https://doi.org/10.1186/1752-153X-6-40
M. Rezayi, M. Ghasemi, R. Karazhian,M. Sookhakian, Y. Alias, Potentiometric Chromate Anion Detection Based on Co (SALEN) 2 Ionophore in a PVC-Membrane Sensor, J. Electrochem. Soc., 161 (2014) B129-B136. https://doi.org/10.1149/2.051406jes
A. Zhuravlev, A. Zacharia, M. Arabadzhi, C. Turetta, G. Cozzi, C. Barbante, Comparison of analytical methods: ICP-QMS, ICP-SFMS and FF-ET-AAS for teh determination of V, Mn, Ni, Cu, As, Sr, Mo, Cd and Pb in ground natural waters, Int. J. Environ. Anal. Chem., 96 (2016) 332–352. https://doi.org/10.1080/03067319.2016.1160380
B. Feist, B. Mikula, Preconcentration of some metal ions with lanthanum-8-hydroxyquinoline co-precipitation system, Food Chem., 147 (2014) 225-229. https://doi.org/10.1016/j.foodchem.2013.09.149
A. Habibiyan, M. Ezoddin, N. Lamei, K. Abdi, M. Amini, M. Ghazi-khansari, Ultrasonic assisted switchable solvent based on liquid phase microextraction combined with micro sample injection flame atomic absorption spectrometry for determination of some heavy metals in water, urine and tea infusion samples, J. Mol. Liq., 242 (2017) 492–496. https://doi.org/10.1016/j.molliq.2017.07.043
Q. Xiong, Y. Lin, W. Wu, J. Hu, Y. Li, K. Xu, Chemometric intraregional discrimination of Chinese liquors based on multi-element determination by ICP-MS and ICP-OES, Appl. Spectrosc. Rev., 56 (2021) 115-127. https://doi.org/10.1080/05704928.2020.1742729.
EH. Evans, J. Pisonero, C.M. Smith, R.N. Taylor, Atomic spectrometry update: review of advances in atomic spectrometry and related techniques, J. Anal. At. Spectrom., 35 (2020) 830–51. https://doi.org/10.1039/d0ja90015j
Supplementary References****************
M. Rezayi, A. Kassim, S. Ahmadzadeh, N.A. Yusof, A. Naji, H. Abbastabar Ahangar, Conductometric determination of formation constants of tris (2-pyridyl) methylamine and titanium (III) in water-acetonitryl mixture, Int. J. Electrochem. Sci., 6 (2011) 4378-4387. http://www.electrochemsci.org
M. Jóźwiak, L. Madej-Kiełbik, Effect of temperature on the process of complex formation crown ether 15C5 with Na+ in the mixture of water with methanol, J. Chem. Thermodyn., 68 (2014) 303-309. https://doi.org/10.1016/j.jct.2013.09.021
S. Ahmadzadeh, A. Kassim, M. Rezayi, G. Hossein Rounaghi, Thermodynamic study of the complexation of p-Isopropylcalix [6] arene with Cs+ Cation in Dimethylsulfoxide-Acetonitrile Binary Media, Molecules, 16 (2011) 8130-8142. https://doi.org/10.3390/molecules16098130
F.A. Christy, P.S. Shrivastav, Conductometric studies on cation-crown ether complexes: A Review. Crit. Rev. Anal. Chem., 41 (2011) 236-269. https://doi.org/10.1080/10408347.2011.589284
A. Genplot, Data analysis and graphical plotting program for scientist and engineers, Computer Graphic Service, Ltd., Ithaca, NY, 1989. http://www.genplot.com/downloads/winnt/Genplot_Manual.pdf
B.B Petković, M. Milčić, D. Stanković, I. Stambolić, D. Manojlović, V.M. Jovanović, S.P. Sovilj, Complexation ability of octaazamacrocyclic ligand toward Co2+, Ni2+, Cu2+ and Zn2+ metal cations: Experimental and theoretical study, Electrochim. Acta, 89 (2013) 680-687. https://doi.org/10.1016/j.electacta.2012.11.100
V. Gutmann, The donor-acceptor approach to molecular interactions. 1979, New York: Plenum Press. https://doi.org/10.1002/ange.19790910738
T. Sakajiri, H. Yajima, T. Yamamura, Density functional theory study on metal-binding energies for human serum transferrin-metal complexes. ISRN Biophys., 2012 (2012) 1-5. https://doi.org/10.5402/2012/124803
Y. Abdollahi, A. Zakaria, N.A. Sairi, K.A. Matori, H.R. Fard Masoumi, A.R. Sadrolhosseini, H. Jahangirian, Artificial neural network modelling of photodegradation in manganese doped Zinc oxide nano-partcles suspension under visible-light irradiation, Sci. World J., 2014 (2014) 726101. https://doi.org/10.1155/2014/726101
M. Rezayi, R. Karazhian, Y. Abdollahi, L. Narimani, S.B. Tavakoly Sany, S. Ahmadzadeh, Y. Alias, Titanium (III) cation selective electrode based on synthesized tris (2pyridyl) methylamine ionophore and its application in water samples, Sci. Reports, 4 (2014) 4664. https://doi.org/10.1038/srep04664
Y. Abdollahi, A. Zakaria, M. Abbasiyannejad, H.R. Fard Masoumi, M. Ghaffari Moghaddam, K.A. Matori, H. ahangirian, A. Keshavarzi, Artificial neural network modeling of p-cresol photodegradation. Chem. Cent. J., 7 (2013) 96. https:// doi.org/10.1186/1752-153X-7-96
Copyright (c) 2022 Analytical Methods in Environmental Chemistry Journal

This work is licensed under a Creative Commons Attribution 4.0 International License.
JOURNAL PUBLISHING AGREEMENT
PLEASE PROVIDE US THE FOLLOWING INFORMATION,
Article entitled:
Corresponding author:
To be published in the journal:
Your Status
I am the sole author of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
I am one author signing on behalf of all co-authors of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
Please tick the above blanks (as appropriate), review the Journal Publishing Agreement, and then sign and date the document in black ink.
Published Journal Article: the author may share a link to the formal publication through the relevant DOI. Additionally theses and dissertations which contain embedded Published Journal Articles as part of the formal submission may be hosted publicly by the awarding institution with a link to the formal publication through the relevant DOI. Any other sharing of Published Journal Articles is by agreement with the publisher only.
Signed: ______________________________________ Name printed: ___________________________________________
Title and Company (if employer representative): _______________________Date: __________________________________