Response surface modeling for the treatment of methylene blue from aqueous media using electro-Fenton process before determination by UV-VIS spectrometer: Kinetic and degradation mechanism
Volume 5, Issue 02, Pages 39-50, Jun 2022 *** Field: Analytical chemistry
Abstract
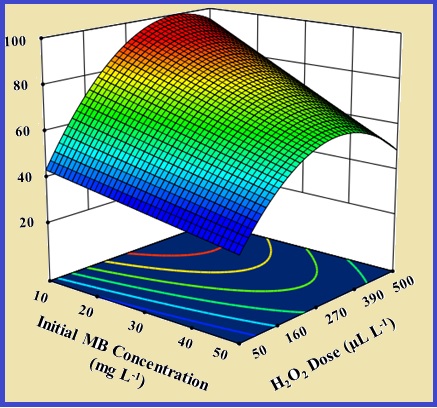
In the present study, response surface methodology was employed to investigate the effects of main variables, including the initial MB concentration, hydrogen peroxide dosage, current density, and electrolysis time on the removal efficiency of MB using the electro-Fenton (EF) process. The MB concentration determination by UV-VIS spectrometer. The EF process degrades the MB contaminant molecules by the highly oxidizing species of the •OH. A quadratic regression model was developed to predict the removal of MB, where the R2 value was found to be 0.9970, which indicates the satisfactory accuracy of the proposed model. ANOVA analysis showed a non-significant lack of fit value (0.0840). Moreover, the predicted correlation coefficient values (R2=0.9915) were reasonably in agreement with the adjusted correlation coefficient value (R2=0.9958), demonstrating a highly significant model for MB dye removal. In addition, the obtained results showed 95.8% MB was removed in the optimum removal efficiency, including the initial MB concentration of 20 mg L-1, H2O2 dosage of 400 μL, and the current density of 7.0 mA cm-2, and electrolysis time of 10 min which was agreed with the predicted removal efficiency of 98.3%. Electrical energy consumption was found to be 0.163 kWh m-3.
References
S. Soni, P. Bajpai, J. Mittal, C. Arora, Utilisation of cobalt doped Iron-based MOF for enhanced removal and recovery of methylene blue dye from waste water, J. Mol. Liq., 314 (2020) 113642. https://doi.org/10.1016/j.molliq.2020.113642.
H.N. Bhatti, Y. Safa, S.M. Yakout, O.H. Shair, M. Iqbal, A. Nazir, Efficient removal of dyes using carboxymethyl cellulose/alginate/polyvinyl alcohol/rice husk composite: adsorption/desorption, kinetics and recycling studies, Int. J. Biol. Macromol., 150 (2020) 861-870. https://doi.org/10.1016/j.ijbiomac.2020.02.093.
J.-C. Lee, H.-J. Kim, H.-W. Kim, H. Lim, Iron-impregnated spent coffee ground biochar for enhanced degradation of methylene blue during cold plasma application, J. Ind. Eng. Chemistry, 98 (2021) 383-388. https://doi.org/10.1016/j.jiec.2021.03.026.
I. Khan, K. Saeed, I. Zekker, B. Zhang, A.H. Hendi, A. Ahmad, S. Ahmad, N. Zada, H. Ahmad, L.A. Shah, Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation, Water, 14 (2022) 242. https://doi.org/10.3390/w14020242.
S.A. Almodaresi, M. Mohammadrezaei, M. Dolatabadi, M.R. Nateghi, Qualitative analysis of groundwater quality indicators based on Schuler and Wilcox diagrams: IDW and Kriging models, J. Environ. Health . Sustain. Dev., 4 (2019) 903 -912. https://doi.org/10.18502/jehsd.v4i4.2023
M.B. Yeamin, M.M. Islam, A.-N. Chowdhury, M.R. Awual, Efficient encapsulation of toxic dyes from wastewater using several biodegradable natural polymers and their composites, J. Clean. Prod., 291 (2021) 125920. https://doi.org/10.1016/j.jclepro.2021.125920.
M. Vakili, M. Rafatullah, B. Salamatinia, A.Z. Abdullah, M.H. Ibrahim, K.B. Tan, Z. Gholami, P. Amouzgar, Application of chitosan and its derivatives as adsorbents for dye removal from water and wastewater: A review, Carbohydrate polymers, 113 (2014) 115-130. https://doi.org/10.1016/j.carbpol.2014.07.007.
A. Kirchon, P. Zhang, J. Li, E.A. Joseph, W. Chen, H.-C. Zhou, Effect of isomorphic metal substitution on the fenton and photo-fenton degradation of methylene blue using Fe-based metal–organic frameworks, ACS Appl. Mater. Interfaces, 12 (2020) 9292-9299. https://doi.org/10.1021/acsami.9b21408.
A. Kausar, S.U. Rehman, F. Khalid, A. Bonilla-Petriciolet, D.I. Mendoza-Castillo, H.N. Bhatti, S.M. Ibrahim, M. Iqbal, Cellulose, clay and sodium alginate composites for the removal of methylene blue dye: Experimental and DFT studies, Int. J. Biol. Macromol., 209 (2022) 576-585. https://doi.org/10.1016/j.ijbiomac.2022.04.044.
A. Bukhari, M. Atta, A. Nazir, M.R. Shahab, Q. Kanwal, M. Iqbal, H. Albalawi, N. Alwadai, Catalytic degradation of MO and MB dyes under solar and UV light irradiation using ZnO fabricated using Syzygium Cumini leaf extract, Zeitschrift für Physikalische Chem., 236 (2022) 659-671. https://doi.org/10.1515/zpch-2021-3096.
A. Syafiuddin, M.A. Fulazzaky, Decolorization kinetics and mass transfer mechanisms of Remazol Brilliant Blue R dye mediated by different fungi, Biotechnol. Reports, 29 (2021) e00573. https://doi.org/10.1016/j.btre.2020.e00573.
M. Ghazaghi, HZ. Mousavi, H. Shirkhanloo, A. Rashidi, Ultrasound assisted dispersive micro solid-phase extraction of four tyrosine kinase inhibitors from serum and cerebrospinal fluid by using magnetic nanoparticles coated with nickel-doped silica as an adsorbent, Microchim. Acta 183 (2016) 2779-2789. https://doi.org/10.1007/s00604-016-1927-z
H. shirkhanloo, M. Osanloo, Nobel method for toluene removal from air based on ionic liquid modified nano-graphen, Int. J. Occup. Hyg., 6 (2014) 1-5. https://ijoh.tums.ac.ir/index.php/ijoh/article/view/89
M.B.H. Abadi, H. Shirkhanloo, J. Rakhtshah, Air pollution control: The evaluation of TerphApm@ MWCNTs as a novel heterogeneous sorbent for benzene removal from air by solid phase gas extraction, Arab. J. Chem., 13 (2020) 1741-1751. https://doi.org/10.1016/j.arabjc.2018.01.011
A. Faghihi-Zarandi, H. Shirkhanloo, C. Jamshidzadeh, A new method for removal of hazardous toluene vapor from air based on ionic liquid-phase adsorbent, Int. J. Environ. Sci. Technol., 16 (2019) 2797-2808. https://doi.org/10.1007/s13762-018-1975-5
C. Jamshidzadeh, H. Shirkhanloo, A new analytical method based on bismuth oxide-fullerene nanoparticles and photocatalytic oxidation technique for toluene removal from workplace air, Anal. Methods Environ. Chem. J., 2 (2019) 73-86. https://doi.org/10.24200/amecj.v2.i01.55
R. Ashouri, H Shirkhanloo, AM Rashidi, SAH Mirzahosseini, N. Mansouri, Dynamic and static removal of benzene from air based on task-specific ionic liquid coated on MWCNTs by sorbent tube-headspace solid-phase extraction procedure, Int. J. Environ. Sci. Technol., 18 (2021) 2377-2390. https://doi.org/10.1007/s13762-020-02995-4
F. Jamali‐Behnam, A.A. Najafpoor, M. Davoudi, T. Rohani‐Bastami, H. Alidadi, H. Esmaily, M. Dolatabadi, Adsorptive removal of arsenic from aqueous solutions using magnetite nanoparticles and silica‐coated magnetite nanoparticles, Environ. Prog. Sustain. Energy, 37 (2018) 951-960. https://doi.org/10.1002/ep.12751.
A. Najafpoor, H. Alidadi, H. Esmaeili, T. Hadilou, M. Dolatabadi, A. Hosseinzadeh, M. Davoudi, Optimization of anionic dye adsorption onto Melia azedarach sawdust in aqueous solutions: effect of calcium cations, Asia‐Pacific J. Chem. Eng., 11 (2016) 258-270. https://doi.org/10.1002/apj.1962.
I. Sirés, E. Brillas, M.A. Oturan, M.A. Rodrigo, M. Panizza, Electrochemical advanced oxidation processes: today and tomorrow. A review, Environ. Sci. Pollut. Res., 21 (2014) 8336-8367. https://doi.org/10.1007/s11356-014-2783-1.
M. Rezayi, L.Y. Heng, A. Kassim, S. Ahmadzadeh, Y. Abdollahi, H. Jahangirian, Immobilization of tris (2 pyridyl) methylamine in a PVC-Membrane Sensor and Characterization of the Membrane Properties, Chem. Cent. J., 6 (2012) 1-6. https://doi.org/10.1186/1752-153X-6-40.
S. Ata, M. Feroz, I. Bibi, I.-u. Mohsin, N. Alwadai, M. Iqbal, Investigation of electrochemical reduction and monitoring of p-nitrophenol on imprinted polymer modified electrode, Synthetic Metals, 287 (2022) 117083. https://doi.org/10.1016/j.synthmet.2022.117083.
Y. Zhu, R. Zhu, Y. Xi, J. Zhu, G. Zhu, H. He, Strategies for enhancing the heterogeneous Fenton catalytic reactivity: a review, Appl. Catal. B: Environ., 255 (2019) 117739. https://doi.org/10.1016/j.apcatb.2019.05.041.
Y.S. Woo, M. Rafatullah, A.F.M. Al-Karkhi, T.T. Tow, Removal of Terasil Red R dye by using Fenton oxidation: a statistical analysis, Desalination Water Treat., 52 (2014) 4583-4591. https://doi.org/10.1080/19443994.2013.804454.
M. Dolatabadi, M.T. Ghaneian, C. Wang, S. Ahmadzadeh, Electro-Fenton approach for highly efficient degradation of the herbicide 2, 4-dichlorophenoxyacetic acid from agricultural wastewater: Process optimization, kinetic and mechanism, J. Mol. Liq., 334 (2021) 116116. https://doi.org/10.1016/j.molliq.2021.116116.
A. Talaiekhozani, M.R. Mosayebi, M.A. Fulazzaky, Z. Eskandari, R. Sanayee, Combination of TiO2 microreactor and electroflotation for organic pollutant removal from textile dyeing industry wastewater, Alexandria Eng. J., 59 (2020) 549-563. https://doi.org/10.1016/j.aej.2020.01.052.
R.A. Khera, M. Iqbal, A. Ahmad, S.M. Hassan, A. Nazir, A. Kausar, H.S. Kusuma, J. Niasr, N. Masood, U. Younas, Kinetics and equilibrium studies of copper, zinc, and nickel ions adsorptive removal on to Archontophoenix alexandrae: conditions optimization by RSM, Desalination Water Treat., 201 (2020) 289-300. https://doi.org/ 10.5004/dwt.2020.25937.
M. Dolatabadi, H. Naidu, S. Ahmadzadeh, A green approach to remove acetamiprid insecticide using pistachio shell-based modified activated carbon; economical groundwater treatment, J. Clean. Prod., 316 (2021) 128226. https://doi.org/10.1016/j.jclepro.2021.128226.
M. López-Guzmán, M.A. Flores-Hidalgo, L. Reynoso-Cuevas, Electrocoagulation process: An approach to continuous processes, reactors design, pharmaceuticals removal, and hybrid systems—A Review, Processes, 9 (2021) 1831. https://doi.org/10.3390/pr9101831.
P. Nidheesh, R. Gandhimathi, Trends in electro-Fenton process for water and wastewater treatment: an overview, Desalination, 299 (2012) 1-15. https://doi.org/10.1016/j.desal.2012.05.011.
E. Brillas, S. Garcia-Segura, Benchmarking recent advances and innovative technology approaches of Fenton, photo-Fenton, electro-Fenton, and related processes: A review on the relevance of phenol as model molecule, Sep. Purif. Technol., 237 (2020) 116337. https://doi.org/10.1016/j.seppur.2019.116337.
J. Casado, Towards industrial implementation of Electro-Fenton and derived technologies for wastewater treatment: A review, J. Environ. Chem. Eng., 7 (2019) 102823. https://doi.org/10.1016/j.jece.2018.102823.
H. Monteil, Y. Pechaud, N. Oturan, M.A. Oturan, A review on efficiency and cost effectiveness of electro-and bio-electro-Fenton processes: application to the treatment of pharmaceutical pollutants in water, Chem. Eng. J., 376 (2019) 119577. https://doi.org/10.1016/j.cej.2018.07.179.
J.J. Rueda-Márquez, I. Levchuk, M. Manzano, M. Sillanpää, Toxicity reduction of industrial and municipal wastewater by advanced oxidation processes (Photo-Fenton, UVC/H2O2, Electro-Fenton and Galvanic Fenton): a review, Catalysts, 10 (2020) 612. https://doi.org/10.3390/catal10060612.
A. Chmayssem, S. Taha, D. Hauchard, Scaled-up electrochemical reactor with a fixed bed three-dimensional cathode for electro-Fenton process: Application to the treatment of bisphenol A, Electrochim. Acta, 225 (2017) 435-442. https://doi.org/10.1016/j.electacta.2016.12.183.
Copyright (c) 2022 Analytical Methods in Environmental Chemistry Journal

This work is licensed under a Creative Commons Attribution 4.0 International License.
JOURNAL PUBLISHING AGREEMENT
PLEASE PROVIDE US THE FOLLOWING INFORMATION,
Article entitled:
Corresponding author:
To be published in the journal:
Your Status
I am the sole author of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
I am one author signing on behalf of all co-authors of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
Please tick the above blanks (as appropriate), review the Journal Publishing Agreement, and then sign and date the document in black ink.
Published Journal Article: the author may share a link to the formal publication through the relevant DOI. Additionally theses and dissertations which contain embedded Published Journal Articles as part of the formal submission may be hosted publicly by the awarding institution with a link to the formal publication through the relevant DOI. Any other sharing of Published Journal Articles is by agreement with the publisher only.
Signed: ______________________________________ Name printed: ___________________________________________
Title and Company (if employer representative): _______________________Date: __________________________________