Adsorption behavior of Crys tal Violet dye in aqueous solution using Co+2 hectorite composite as adsorbent surface
Volume 6, Issue 01, Pages 5-16, March 2023 *** Field: Analytical Chemistry Method
Abstract
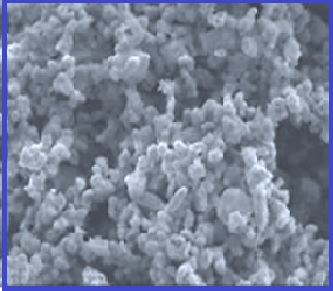
This study focused on the adsorption behavior of the cationic Crystal
Violet (CV) dye from aqueous solutions using a Co+2‒hectorite
composite as an adsorbent surface. The initial and equilibrium CV dye
concentrations were determined using a UV-Vis spectrophotometer. The
results were discussed and presented for the impacts of pH, primary CV
dye concentration, composite dosage, and temperature. The optimum
conditions were found for eliminating Crys tal Violet dye from the
aqueous solution at a pH 4, ideal temperature 293 K, and 0.5 g L-1
of composite dose. The pseudo-second-order kinetic, intraparticle
diffusion analyzed the tes ts’ data and film diffusion models. Each
model’s defining features have been identified, and these models were
in good agreement and in charge of regulating the adsorption reaction.
The adsorption operation was also thermodynamically examined to
determine thermodynamic variables such as Gibbs free energy (ΔGo),
entropy (ΔSo), activation energy (Ea), and enthalpy (ΔHo). The negative
value of Gibbs free energy (ΔGo) and enthalpy (ΔHo) indicated that
the adsorption process was a spontaneous and exothermic reaction.
While the activation energy (Ea) data which fell within the normal
range for physisorption, was showed at 22.434 kJ mol-1.
The physical adsorption occurs between CV dye and
adsorbent .
References
F. Kooli, S. Rakass, Y. Liu, M. Abboudi, H.O. Hassani, S.M. Ibrahim, F. Wadaani, R. Al-Faze, Eosin removal by Cetyl trimethylammonium-Cloisites: influence of the surfactant solution type and regeneration properties, Mol., 24 (2019) 3015. https://doi.org/10.3390/molecules24163015.
F.M. Valadi, A. Ekramipooya, M.R. Gholami, Selective separation of Congo Red from a mixture of anionic and cationic dyes using magnetic-MOF: Experimental and DFT study, J. Mol. Liq., 318 (2020) 114051. https://doi.org/10.1016/j.molliq.2020.114051.
P. S. Kumar, G. J. Joshiba, C. C. Femina, P. Varshini, S. Priyadharshini, M. A. Karthick, R. Jothirani, A critical review on recent developments in the low-cost adsorption of dyes from wastewater, Desalin. Water Treat., 172 (2019) 395-416. https://doi.org/10.5004/dwt.2019.24613.
S. Benkhaya, S. M'rabet, A. El Harfi, Classifications, properties, recent synthesis and applications of azo dyes, Heliyon, 6 (2020) e03271. https://doi.org/10.1016/j.heliyon.2020.e03271.
G. Crini, E. Lichtfouse, Advantages and disadvantages of techniques used for wastewater treatment, Environ. Chem. Lett., 17 (2019) 145-155. https://doi.org/10.1007/s10311-018-0785-9.
S. Samsami, M. Mohamadizaniani, M.H. Sarrafzadeh, E.R. Rene, M. Firoozbahr, Recent advances in the treatment of dye-containing wastewater from textile industries: Overview and perspectives, Proc. Safe. Environ. Prot., 143 (2020) 138-163. https://doi.org/10.1016/j.psep.2020.05.034.
E. Routoula, S.V. Patwardhan, Degradation of anthraquinone dyes from effluents: a review focusing on enzymatic dye degradation with industrial potential, Environ. Sci. Technol., 54 (2020) 647-664. https://doi.org/10.1021/acs.est.9b03737.
A. Tkaczyk, K. Mitrowska, A. Posyniak, Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review, Sci. Total Environ., 717 (2020) 137222. https://doi.org/10.1016/j.scitotenv.2020.137222.
L. Largitte, R. Pasquier, A review of the kinetics adsorption models and their application to the adsorption of lead by an activated carbon, Chem. Eng. Res. Desi., 109 (2016) 495-504. https://doi.org/10.1016/j.cherd.2016.02.006.
C. Santhosh, V. Velmurugan, G. Jacob, S.K. Jeong, A.N. Grace, A. Bhatnagar, Role of nanomaterials in water treatment applications: a review, Chem. Eng. J., 306 (2016) 1116-1137. https://doi.org/10.1016/j.cej.2016.08.053.
M. Berradi, R. Hsissou, M. Khudhair, M. Assouag, O. Cherkaoui, A. El Bachiri, A. El Harfi, Textile finishing dyes and their impact on aquatic environs, Heliyon, 5 (2019) e02711. https://doi.org/10.1016/j.heliyon.2019.e02711.
M.T. Yagub, T.K. Sen, S. Afroze, H.M. Ang, Dye and its removal from aqueous solution by adsorption: a review, Adv. Coll. Inter. Sci., 209 (2014) 172-184. https://doi.org/10.1016/j.cis.2014.04.002.
T.A. Saleh, Protocols for synthesis of nanomaterials, polymers, and green materials as adsorbents for water treatment technologies, Environ. Tech. Innov., 24 (2021) 101821. https://doi.org/10.1016/j.eti.2021.101821.
X. Liu, H. Pang, X. Liu, Q. Li, N. Zhang, L. Mao, M. Qiu, B. Hu, H. Yang, X. Wang, Orderly porous covalent organic frameworks-based materials: superior adsorbents for pollutants removal from aqueous solutions, Innovation, 2 (2021) 100076. https://doi.org/10.1016/j.xinn.2021.100076.
I.C. Ossai, A. Ahmed, A. Hassan, F.S. Hamid, Remediation of soil and water contaminated with petroleum hydrocarbon: A review, Environ. Tech. Innov., 17 (2020) 100526. https://doi.org/10.1016/j.eti.2019.100526.
J. Liu, G. Zhang, Recent advances in synthesis and applications of clay-based photocatalysts: a review, Phys. Chem. Chem. Phys., 16 (2014) 8178-8192. https://doi.org/10.1039/C3CP54146K.
Z. Wang, Z. Wang, S. Lin, H. Jin, S. Gao, Y. Zhu, J. Jin, Nanoparticle-templated nanofiltration membranes for ultrahigh performance desalination, Nat. comm., 9 (2018) 1-9. https://doi.org/10.1038/s41467-018-04467-3.
D. Yue, Y. Jing, J. Ma, C. Xia, X. Yin, Y. Jia, Removal of neutral red from aqueous solution by using modified hectorite, Desalination, 267 (2011) 9-15. https://doi.org/10.1016/j.desal.2010.08.038
N.U.M. Nizam, M.M. Hanafiah, E. Mahmoudi, A.A. Halim, A.W. Mohammad, The removal of anionic and cationic dyes from an aqueous solution using biomass-based activated carbon, Sci. Rep., 11 (2021) 1-17. https://doi.org/10.1038/s41598-021-88084-z
C.F. Mok, Y.C. Ching, F. Muhamad, N.A. Abu Osman, N.D.Hai, C.R. Che Hassan, Adsorption of dyes using poly (vinyl alcohol)(PVA) and PVA-based polymer composite adsorbents: a review, J. Poly. Environ., 28 (2020) 775-793. https://doi.org/10.1007/s10924-020-01656-4
I. Chaari, E. Fakhfakh, M. Medhioub, F. Jamoussi, Comparative study on adsorption of cationic and anionic dyes by smectite rich natural clays, J. Mol. Struct., 1179 (2019) 672-677. https://doi.org/10.1016/j.molstruc.2018.11.039
A.J. Ibrahim, ZnO nanostructure synthesis for the photocatalytic degradation of azo dye methyl orange from aqueous solutions utilizing activated carbon, Anal. Meth. Environ. Chem. J., 5 (2022) 5-19. https://doi.org/10.24200/amecj.v5.i04.200
A.N.M.A. Haque, R. Remadevi, O.J. Rojas, X. Wang, M. Naebe, Kinetics and equilibrium adsorption of methylene blue onto cotton gin trash bioadsorbents, Cellulose, 27 (2020) 6485-6504. https://doi.org/10.1007/s10570-020-03238-y
A.J. Ibrahim, Ultraviolet-activated sodium perborate process (UV/SPB) for removing humic acid from water, Anal. Meth. Environ. Chem. J., 5 (2022) 5-18. https://doi.org/10.24200/amecj.v5.i03.191
Y.M. Vargas-Rodríguez, A. Obaya, J.E. García-Petronilo, G.I. Vargas-Rodríguez, A. Gómez-Cortés, G. Tavizón, J.A. Chávez-Carvayar, Adsorption studies of aqueous solutions of methyl green for halloysite nanotubes: Kinetics, isotherms, and thermodynamic parameters, Amer. J. Nanom., 9 (2021) 1-11. http://pubs.sciepub.com/ajn/9/1/1
J.A. Naser, T.A. Himdan, A.J. Ibraheim, Adsorption kinetic of malachite green dye from aqueous solutions by electrospun nanofiber Mat, Orient. J. Chem., 33 (2017) 3121-3129. http://dx.doi.org/10.13005/ojc/330654
A.B. Fradj, A. Boubakri, A. Hafiane, S.B. Hamouda, Removal of azoic dyes from aqueous solutions by chitosan enhanced ultrafiltration, Res. Chem., 2 (2020) 100017. https://doi.org/10.1016/j.rechem.2019.100017
C. Xia, Y. Jing, Y. Jia, D. Yue, J. Ma, X. Yin, Adsorption properties of congo red from aqueous solution on modified hectorite: Kinetic and thermodynamic studies, Desalination, 265 (2011) 81-87. https://doi.org/10.1016/j.desal.2010.07.035
B. Obradović, Guidelines for general adsorption kinetics modeling, Hemi. Ind., 74 (2020) 65-70. https://doi.org/10.2298/HEMIND200201006O
Y. Kuang, X. Zhang, S. Zhou, Adsorption of methylene blue in water onto activated carbon by surfactant modification, Water, 12 (2020) 587. https://doi.org/10.3390/w12020587
S. Debnath, U.C. Ghosh, Kinetics, isotherm and thermodynamics for Cr(III) and Cr(VI) adsorption from aqueous solutions by crystalline hydrous titanium oxide, J. Chem. Therm., 40 (2008) 67-77. https://doi.org/10.1016/j.jct.2007.05.014
A.S. Özcan, B. Erdem, A. Özcan, Adsorption of Acid Blue 193 from aqueous solutions onto Na–bentonite and DTMA–bentonite, J. Coll. Inter. Sci., 280 (2004) 44-54. https://doi.org/10.1016/j.jcis.2004.07.035
Copyright (c) 2023 Analytical Methods in Environmental Chemistry Journal

This work is licensed under a Creative Commons Attribution 4.0 International License.
JOURNAL PUBLISHING AGREEMENT
PLEASE PROVIDE US THE FOLLOWING INFORMATION,
Article entitled:
Corresponding author:
To be published in the journal:
Your Status
I am the sole author of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
I am one author signing on behalf of all co-authors of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
Please tick the above blanks (as appropriate), review the Journal Publishing Agreement, and then sign and date the document in black ink.
Published Journal Article: the author may share a link to the formal publication through the relevant DOI. Additionally theses and dissertations which contain embedded Published Journal Articles as part of the formal submission may be hosted publicly by the awarding institution with a link to the formal publication through the relevant DOI. Any other sharing of Published Journal Articles is by agreement with the publisher only.
Signed: ______________________________________ Name printed: ___________________________________________
Title and Company (if employer representative): _______________________Date: __________________________________