Analytical study on lead elimination by anionic clays: Characterization, adsorption kinetics, isotherm, thermodynamic, mechanism and adsorption
Volume 6, Issue 03, Pages 67-88, Sep 2023 *** Field: Analytical Environmental Chemistry
Abstract
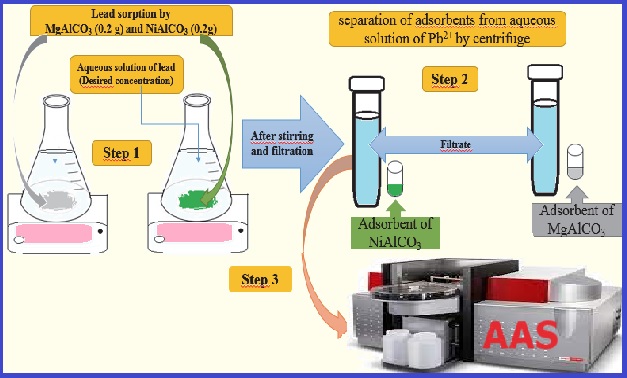
The co-precipitation method synthesized the synthetic anionicMg–Al and Ni-Al clays with three molar ratios (Mg/Al, Ni/Al). The samples were characterized by powder XRD, Fourier transform infrared spectroscopy (FTIR), and scanning electron microscopy (SEM). No other crystalline phases were detected in the powder XRD patterns of the co-precipitated samples. The infrared spectra obtained all the functional groups that characterize these two types of anionic clays. SEM micrographs indicate the presence of particles and aggregates. The particles, or aggregates, are in the form of plates, supported by particles of acceptable sizes. The optimal pH for maximum lead adsorption is about 6.5 for both clays. The optimal adsorbent masses for the maximum percentages of lead removal are 0.2 g for Mg3AlCO3 and 0.25 g for Ni3AlCO3. The Mg3AlCO3 has a maximum adsorption capacity of lead, where qm=73.42 mg g-1. The adsorbed amount increases with increasing temperature for both types of clays studied. The equilibrium time of Pb2+ adsorption is reached after 5 min for both clays. The most appropriate models to describe the experimental data of adsorption kinetics and isotherms are pseudo-second-order and Langmuir. The detection limit (LOD) was 0.272 mg L-1. The linearity range was 1 to 5 mg L-1(R2 0.9997).
References
T.V. Toledo, C.R. Bellato, K.D. Pessoa, M.P. Fontes, Magnetic compounds based in hydrotalcites for removal of anionic contaminants in water, Quim. Nova., 36 (2013) 419–425. http://doi.org/10.1590/S0100-40422007000500005.
M. Bouraada, F. Belhalfaoui, M.S. Ouali, Sorption study of an acid dye from an aqueous solution on modified Mg–Al layered double hydroxides, J. Hazard. Mater., 163 (2009) 463–467. http://doi.org/ 10.1016/j.jhazmat.2008.06.108
L. D. Miranda, C. R., Bellato, M. P. Fontes, M. F. de Almeida, J. L. Milagres, &L. A. Minim, Preparation and evaluation of hydrotalcite-iron oxide magnetic organocomposite intercalated with surfactants for cationic methylene blue dye removal, Chem. Eng. J., 254 (2014) 88-97. http://doi.org/10.1016/j.cej.2014.05.094
R. Shi, P. Yangn, Y. Yin, X. Dong, J. Li. Fabrication of porous microspheres and network arrays of Zn–Al hydrotalcite-like compounds on Al substrate via facile hydrothermal method, Ceram. Int., 40 (2014) 6855–6863. http://doi.org/10.1016/j.ceramint.2013.12.005.
Esthela Ramos-Ramírez, Norma L Gutiérrez Ortega, Cesar A Contreras Soto, Maria T Olguín Gutiérrez. Adsorption isotherm studies of chromium (VI) from aqueous solutions using sol–gel hydrotalcite-like compounds, J. Hazard. Mater., 172 (2009) 1527–1531. http://doi.org/10.1016/j.jhazmat.2009.08.023
S. V. Prasanna, P. V. Kamath, Anion-exchange reactions of layered double hydroxides: interplay between coulombic and H-bonding interactions, Ind. Eng. Chem. Res., 48 (2009) 6315-6320. http://doi.org/10.1021/ie9004332
A. V. Radha, P. V. Kamath, C. Shivakumara, Mechanism of the anion exchange reactions of the layered double hydroxides (LDHs) of Ca and Mg with Al, Solid State Sci., 7 (2005) 1180-1187. http://doi.org/10.1016/j.solidstatesciences.2005.05.004
M. Lakraimi, A. Legrouri, A. Barroug, A. DeRoy and J. P. Besse, Preparation of a new stable hybrid material by chloride–2,4-dichlorophenoxyacetate ion exchange into the zinc–aluminium–chloride layered double hydroxide, J. Mater. Chem., 10 (2000) 1007-1011. http://doi.org/10.1039/A909047I
N. Drici. Hydroxydes doubles lamellaires, synthèse, caractérisation et propriétés. Matériaux. Université Sorbonne Paris Cité, Français, NNT : 2015USPCD007, 2015. https://theses.hal.science/tel-01488539v1/file/edgalilee_th_2015_drici.pdf
E. Géraud, V. Prévot, J. Ghanbaja, F. Leroux, Macroscopically ordered hydrotalcite-type materials using self-assembled colloidal crystal template, Chem. Mater., 18 (2006) 238–240. http://doi.org/10.1021/cm051770i
K. Kadirvelu, M. Kavipriya, C. Karthika, M. Radhika, N. Vennilamani, S. Pattabhi, Utilization of various agricultural wastes for activated carbon preparation and application for the removal of dyes and metal ions from aqueous solutions, Bioresour. Technol., 87 (2003) 129-32. http://doi.org/10.1016/s0960-8524(02)00201-8.
A. K. Jain, V. K. Gupta, A. Bhatnagar, Utilization of industrial waste products as adsorbents for the removal of dyes, J. Hazard. Mater., 101 (2003) 31-42. http://doi.org/10.1016/s0304-3894(03)00146-8.
Y. Yasin, M. Mohamad, A. Saad, A. Sanusi, F. H. Ahmad, Removal of lead ions from aqueous solutions using intercalated tartrate-Mg–Al layered double hydroxides, Desalin. Water Treatment., 52 (2014) 4266-4272. http://doi.org/10.1080/19443994.2013.803935
S. Yanming, L. Dongbin, L. Shifeng, F. Lihui, C. Shuai, M. A. Haque, Removal of lead from aqueous solution on glutamate intercalated layered double hydroxide, Arab. J. Chemy., 10 (2017) S2295-S2301. http://doi.org/10.1016/j.arabjc.2013.08.005.
E. J. Pacer, C. D. Palmer, P. J. Parsons, Determination of lead in blood by graphite furnace atomic absorption spectrometry with Zeeman background correction: Improving a well-established method to support a lower blood lead reference value for children, Spectrochim. Acta Part B: Atom. Spect., 190 (2022) 106324. http://doi.org/10.1016/j.sab.2021.106324
M. S. Tudosie, G. Caragea, D. M. Popescu, O. Avram, D. Serban, C. G. Smarandache, A. M. Dascalu, Optimization of a GF AAS method for lead testing in blood and urine: A useful tool in acute abdominal pain management in emergency, Exp. Ther. Med., 22 (2021) 1-8. http://doi.org/10.3892/etm.2021.10417.
ZW. Zhang, S. Shimbo, N. Ochi, M. Eguchi, T. Watanabe, CS. Moon, M. Ikeda, Determination of lead and cadmium in food and blood by inductively coupled plasma mass spectrometry: a comparison with graphite furnace atomic absorption spectrometry, Sci. Total Environ., 205 (1997) 179-87. http://doi.org/10.1016/s0048-9697(97)00197-6.
M. Trzcinka-Ochocka, R. Brodzka, B. Janasik, Useful and fast method for blood lead and cadmium determination using ICP-MS and GF-AAS; validation parameters, J. Clin. Lab. Anal., 30 (2016) 130-9. http://doi.org/10.1002/jcla.21826.
D. Fuger, Mise en application de processus analytique complexe Analyse de métaux par ICP-AES. Robert Schuman–Département chimie, Université de Strasbourg, France, (2011). https://www.chemphys.fr/mpb/teach/ICP-AES/ICP-AES.pdf
R. Riyanto, Determination of lead in waste water using cyclic voltammetry by platinum wire electrode, J. Sci. Data Anal., 14 (2014) 22-33. http://doi.org/10.20885/eksakta.vol14.iss2.art3.
F. Delorme, A. Seron, M. Bizi, V. Jean-Prost, D. Martineau. Effect of time on the reconstruction of the Mg4Al2(OH)12CO3.3H2O layered double hydroxide in a Na2CO3 solution, J. Mater. Sci., 41 (2006) 4876-4882. http://doi.org/10.1007/s10853-006-0304-4
K. Klemkaite, I. Prosycevas, R. Taraskevicius, A. Khinsky, A. Kareiva, Synthesis and characterization of layered double hydroxides with different cations (Mg, Co, Ni, Al), decomposition and reformation of mixed metal oxides to layered structures, Open Chem., 9 (2011) 275-282. http://doi.org/10.2478/s11532-011-0007-9
A. Faour, C. Mousty, V. Prevot, B. Devouard, A. De Roy, P. Bordet, C. Taviot-Gueho, Correlation among structure, microstructure, and electrochemical properties of NiAl–CO3 layered double hydroxide thin films, J. Phys. Chem. C, 116 (2012) 15646-15659. http://doi.org/10.1021/jp300780w
F. Cavani, F. Trifiro, A Vaccari, Hydrotalcite-type anionic clays: Preparation, properties and applications, Catal. Today, 11 (1991) 173-301. http://doi.org/10.1016/0920-5861(91)80068-K
L. Xuefeng, H. Wanguo, X. Yingming, Sorption of lead ion by layered double hydroxide intercalated with diethylenetriaminepentaacetic acid, Colloids Surf. A Physicochem. Eng. Asp., 366 (2010) 50-57. http://doi.org/10.1016/j.colsurfa.2010.05.012
D. Zhao, G. Sheng, J. Hu, C. Chen, X. Wang, The adsorption of Pb (II) on Mg2Al layered double hydroxide, Chem, Eng, J., 171 (2011) 167-174. http://doi.org/10.1016/j.cej.2011.03.082.
M. Arshadi, M. J. Amiri, S. Mousavi, Kinetic, equilibrium and thermodynamic investigations of Ni (II), Cd (II), Cu (II) and Co (II) adsorption on barley straw ash, Water Resour. Ind., 6 (2014) 1-17. http://doi.org/10.1016/j.wri.2014.06.001
J. B. Huo, G. Yu, Layered double hydroxides derived from MIL-88A (Fe) as an efficient adsorbent for enhanced removal of lead (II) from water, Int. J. Mol. Sci., 23 (2022) 14556. http://doi.org/10.3390/ijms232314556
G. W. Kajjumba, S. Emik, A. Öngen, H. K. Özcan, S. Aydın, Modelling of adsorption kinetic processes—errors, theory and application, Adv. Sorp. process applications, (2018) 1-19. http://doi.org/10.5772/intechopen.80495
C. H. Giles, T. H. MacEwan, S. N. Nakhwa, D. Smith, Studies in adsorption. Part XI. A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids, J. Chem. Soc. Resumed., 32 (1960) 3973–3993. https://doi.org/10.1039/JR9600003973
V. Rives, Layered double hydroxides: present and future, Nova Science Publishers, Inc., New York, 2001, http://doi.org/10.1016/S0169-1317(02)00112-6
R. Marangoni, M. Bouhent, , C. Taviot-Gueho, F. Wypych, F. Leroux. Zn2Al layered double hydroxides intercalated and adsorbed with anionic blue dyes: A physico-chemical characterization, J. Colloid Interface Sci., 333 (2009) 120-127. http://doi.org/10.1016/j.jcis.2009.02.001
T.N. Moroz Formation of spinel from hydrotalcite-like minerals and destruction of chromite implanted by inorganic salts, App. Clay Sci., 18 (2001) 29–36. http://doi.org/10.1016/S0169-1317(00)00027-2
P. Benito, I. Guinea, F. M. Labajos, V. Rives, Microwave-assisted reconstruction of Ni, Al hydrotalcite-like compounds, J. Solid State Chem., 181 (2008) 987-996. http://doi.org/10.1016/j.jssc.2008.02.003
A. A. Inyinbor, F. A. Adekola, G. A. Olatunji, Kinetics, isotherms and thermodynamic modeling of liquid phase adsorption of Rhodamine B dye onto Raphia hookerie fruit epicarp, Water Resour. Ind., 15 (2016) 14-27. http://doi.org/10.1016/j.wri.2016.06.001
J. X. Lin, S. L. Zhan, M. H. Fang, X. Q. Qian, H. Yang, Adsorption of basic dye from aqueous solution onto fly ash, J. Environ. Manage., 87 (2008) 193-200. http://doi.org/10.1016/j.jenvman.2007.01.001
B. Houari, S. Louhibi, K. Tizaoui, L. Boukli-hacene, B. Benguella, T. Roisnel, V. Dorcet, New synthetic material removing heavy metals from aqueous solutions and wastewater, Arab. J. Chem., 12 (2019) 5040-5048. http://doi.org/10.1016/j.arabjc.2016.11.010
N. Ayawei, C. Y. Abasi, D. Wankasi, E. D. Dikio, Layered double hydroxide adsorption of lead: equilibrium, thermodynamic and kinetic studies, Int. J. Adv. Res. Chem. Sci., 2 (2015) 22-32. https://www.arcjournals.org/pdfs/ijarcs/v2-i5/3.pdf
Copyright (c) 2023 Analytical Methods in Environmental Chemistry Journal

This work is licensed under a Creative Commons Attribution 4.0 International License.
JOURNAL PUBLISHING AGREEMENT
PLEASE PROVIDE US THE FOLLOWING INFORMATION,
Article entitled:
Corresponding author:
To be published in the journal:
Your Status
I am the sole author of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
I am one author signing on behalf of all co-authors of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
Please tick the above blanks (as appropriate), review the Journal Publishing Agreement, and then sign and date the document in black ink.
Published Journal Article: the author may share a link to the formal publication through the relevant DOI. Additionally theses and dissertations which contain embedded Published Journal Articles as part of the formal submission may be hosted publicly by the awarding institution with a link to the formal publication through the relevant DOI. Any other sharing of Published Journal Articles is by agreement with the publisher only.
Signed: ______________________________________ Name printed: ___________________________________________
Title and Company (if employer representative): _______________________Date: __________________________________