Air pollution Analysis: Nickel paste on Multi-walled carbon nanotubes as novel adsorbent for the mercury removal from air
Vol 2, Issue 03, Pages 79-88,*** Field: Air Pollution Analysis
Abstract
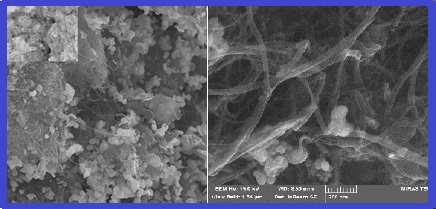
Mercury as a hazardous material caused health problem in humans.In this study,mercury vapor removed from air by nickel-coated on multi-walled carbon nanotubes(Ni-MWCNTs)as a novel sorbent.Amalgamation of mercury with Ni-MWCNTs was achieved by solid-gas phase removal method(SGPR).In bench scale set up, the mercury vapor generated and moved to sorbent at optimized flow rate.After thermal desorption of Ni-MWCNTs at 200oC, the mercury vapor flowed to quartz glass cell with argon gas and determined by cold vapor atomic absorption spectrometer technique(CV-AAS).In optimized conditions,25 mg of Ni-MWCNTs and MWCNTs with different size from 30-100nm was used.The adsorption capacity of sorbents was obtained 194 mg g-1 and 64 mg g-1,respectively.The efficient recovery was obtained at optimized conditions such as, temperature of 25-40 and flow rate of 200 mL min-1.So, Ni-MWCNTs had good potential for removal of mercury vapor from the air and can be used as a low cost and efficient sorbent in industrial workplace
References
[[1] A. Wilk, E. Kalisińska, D. I. Kosik-Bogacka, Cadmium, lead and mercury concentrations in pathologically altered human kidneys, Environ. Geochem. Health, 39 (2017) 889–899.
B. Mansouri, R. Baramaki, M. Ebrahimpour, Acute toxicity bioassay of mercury and silver on Capoeta fusca (black fish), Toxicol. Indus. Health, 28 (2012) 393-398.
M. Gochfeld, Cases of mercury exposure, bioavailability, and absorption, Ecotoxicol. Environ. Saf. J., 56 (2003) 174-179.
R. Afrin, M. Y. Mia, M. A. Ahsan, A. Akbor, Concentration of heavy metals in available fish species in the Turag river, Bangladesh, Pakistan, J. Sci. Ind. Res., Series B: Biol. Sci., 58 (2015)104–110.
S. Cwan, Occupational toxicology, CRC Press. (2003) 317-319.
PB. Tchounwou, WK. Ayensu, N. Ninashvili, D. Sutton, Environmental exposure to mercury and its toxicopathologic implications for public health, Environ. Toxicol. J., 18 (2003) 149-175.
H. Shirkhanloo, M. Osanloo, M. Ghazaghi, H. Hassani, Validation of a new and cost-effective method for mercury vapor removal based on silver nanoparticles coating on micro glassy balls, Atmos. Pollut. Res., 8 (2017) 359-365
NIOSH Manual of analytical methods (NMAM, 5 Edition), U.S. department of health and human services, 2017.
Occupational Safety and Health Administration (OSHA), Air contaminants, 29 CFR 1910.1000,2017.
http://www.osha.gov/laws- regs/regulations/standardnumber/1910/1910.1000.
M. Marczak, S. Budzyń, J. Szczurowski, K. Kogut, P. Burmistrz, Active methods of mercury removal from flue gases, Environ. Sci. Pollut. Res. Int., 26 (2019) 8383-8392.
JG. Evan, WP. Henry, AH. Richard, Novel sorbents for mercury removal from flue gas, Ind. Eng. Chem. Res., 39 (2000) 1020-1029.
Wu. Xiang, D. Yufeng, Li. Na, Hu. Peng, Y.Ting, M. Jialin, R. Shaojun, W. Hongqi, Regenerable Ce–Mn/TiO2 catalytic sorbent for mercury removal with high resistance to SO2. Energ. Fuel., 33(2019) 8835-8842.
M. Lingjun, Y. Caixia, Y. Liang, H. Qihuang, H. Lina, C Liping, B Weiren, W Jiancheng, Effects of supports on Pd–Fe bimetallic sorbents for Hg0 removal activity and regeneration performance from coal-derived fuel gas, Energ. Fuel., 33 (2019) 8976-8984.
Z. Xiaoyang, C. Lin, L. Yuzhong, Z. Yongchun, D Yong, C Shensong, Adsorption and oxidation of mercury by montmorillonite powder modified with different copper compounds, Energ. Fuel., 33 (2019) 7852-7860.
M. Jian, W. Chang, K. Lingnan, L. Xiaoli, H, Qixing, Z Hui, Y. Shijian, Outstanding performance of recyclable amorphous MoS3 supported on TiO2 for capturing high concentrations of gaseous elemental mercury: mechanism, kinetics, and application., Environ. Sci. Technol., 53 (2019) 4480-4489.
F. Seef Saadi, KA. Mohamed, J. Wan Zurina Binti, A. Mohammed Abdulhakim A, F. Sabah Saadi, K. Suhana Binti, L. Sai Hin, C. Ming Fai, A. Ali Najah, ES. Ahmed, Artificial neural network approach for modelling of mercury ions removal from water using functionalized CNTs with deep eutectic solvent, Int. J. Mol. Sci., 17( 2019) 4206-4212.
S.S. Fiyadh, M.A. AlSaadi, .M.K AlOmar, S.S. Fayaed, A.R. Hama, S. Bee, A. El-Shafie, The modelling of lead removal from water by deep eutectic solvents functionalized CNTs: Artificial neural network (ANN) approach, Water Sci. Technol., 76( 2017) 2413–2426.
S.S. Fiyadh, M.A. AlSaadi, .M.K AlOmar, S.S. Fayaed, A. El-Shafie, Lead removal from water using DES functionalized CNTs: ANN modeling approach. Desalin. Water Treat., 150(2019) 105-113.
M.A. AlSaadi, A. Al Mamun A., Alam M.Z., Amosa M.K., Atieh M.A. Removal of cadmium from water by CNT–PAC composite: Effect of functionalization, Nano., 11(2016) 1650011.
M. Lu M, T, Ohba, K. Kaneko, K. Hata, M. Yumura, S. Iijima, H. Komatsu, A. Sakuma, H. Kanoh, Electron density modification of single wall carbon nanotubes (SWCNT) by liquid-phase molecular adsorption of hexaiodobenzene., Materials., 6(2013) 535–543.
R. Yan, D.T. Liang, J.H. Tay, Control of mercury vapor emissions from combustion flue gas. Environ. Sci. Pollut. Res., J., 10 (2003) 399-407.
PM. Ajayan, O.Z. Zhou, Applications of carbon nanotubes, Carbon Nanotubes J., 80 (2001) 391-425.
P.E. Díaz-Flores, J.A. Arcibar-Orozco, N.V. Perez-Aguilar, J.R. Rangel-Mendez, V.M. Ovando Medina, J.A. Alcalá-Jáuegui, Adsorption of organic compounds onto multiwall and nitrogen-doped carbon nanotubes: Insights into the adsorption mechanisms, Water, Air Soil Pollut. J., 228 (2017) 133- 147.
J. Xu, Z. Cao, Y. Zhang, Z. Yuan, Z. Lou, X. Xu, X. Wang, A review of functionalized carbon nanotubes and graphene for heavy metal adsorption from water: Preparation, application, and mechanism, Chemosphere. J., 195 (2018) 351- 364.
W.N. Nyairo, Y. Ramazan Eker, C. Kowenje, I. Akin, H. Bingol, A. Tor, D.M. Ongeri. Efficient adsorption of lead (II) and copper (II) from aqueous phase using oxidized multiwalled carbon nanotubes/polypyrrole composite, Sep. Scie. Technol. J., 52(2018) 1498-1510.
O. Moradi, K. Zare, M. Yari, Interaction of some heavy metal ions with single walled carbon nanotube, Int. J. Nano Dimens., 1 (2011) 203-20.
F. Su, C. Lu, S. Hu, Adsorption of benzene, toluene, ethylbenzene and p-xylene by NaOCl-oxidized carbon nanotubes, Colloids Surf., A Physicochem. Eng. Asp., 353 (2010) 83-91.
A. Safavi, N. Maleki, M.M. Doroodmand, Fabrication of a selective mercury sensor based on the adsorption of cold vapor of mercury on carbon nanotubes: Determination of mercury in industrial wastewater, J. Hazard. Mater., 173 (2010) 622-9.
C.K Kuo, Desorption and re-adsorption of carbon nanotubes: Comparissons of sodium hydroxiid and microwave irradiation processes, J. Hazard. Mater., 152 (2008) 949-54.
E. Granite, H. Pennline, R. Hargis, Novel sorbents for mercury removal from flue gas, Ind. Eng. Chem. Res., J., 39 (2000) 1020-1029.
S.E. Lindberg, W.J, Stratton, Atmospheric mercury speciation, concentrations and behavior of reactive gaseous mercury in ambient air, Environ. Sci. Technol., 32 (1998) 49-57.
T.G. Lee, Comprison of Hg captuer efficiencies of three in situ generated sorbent, Environ. Energy Eng., AIChE J., 47 (2001) 954-961.
Copyright (c) 2019 Analytical Methods in Environmental Chemistry Journal

This work is licensed under a Creative Commons Attribution 4.0 International License.
JOURNAL PUBLISHING AGREEMENT
PLEASE PROVIDE US THE FOLLOWING INFORMATION,
Article entitled:
Corresponding author:
To be published in the journal:
Your Status
I am the sole author of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
I am one author signing on behalf of all co-authors of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
Please tick the above blanks (as appropriate), review the Journal Publishing Agreement, and then sign and date the document in black ink.
Published Journal Article: the author may share a link to the formal publication through the relevant DOI. Additionally theses and dissertations which contain embedded Published Journal Articles as part of the formal submission may be hosted publicly by the awarding institution with a link to the formal publication through the relevant DOI. Any other sharing of Published Journal Articles is by agreement with the publisher only.
Signed: ______________________________________ Name printed: ___________________________________________
Title and Company (if employer representative): _______________________Date: __________________________________