A Nickel separation from human blood samples based on Amine and Amide Functionalized magnetic graphene oxide nano structure by dispersive sonication micro solid phase extraction
Volume 3, Issue 01, Pages 5-16, Mar 2020 *** Field: Human Bio-nanotechnology
Abstract
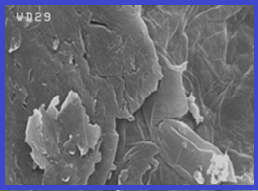
Nickel (Ni) is toxic effect on human body and must be determined in human blood samples. In this study, Ni ions separated and preconcentrated from blood samples based on magnetic Fe3O4-supported amine/amide-functionalized graphene oxide (Fe3O4@A/A-GO) nanoparticles by dispersive sonication micro solid phase extraction (DS- μ-SPE). By procedure, 10 mg of Fe3O4@A/A-GO was dispersed in 10 mL of human blood samples with sonication for 5.0 min and then separated from liquid phase with magnetic accessory. The Ni ions was extracted based on amine/amide covalence bonding of Fe3O4@A/A-GO sorbent (Ni---: NH2). Then, the Ni ions back-extracted from Fe3O4@A/A-GO in low pH with nitric acid (0.2 mL, 0.3 M) which was diluted with DW up to 0.5 mL and finally, was determined by ET-AAS(peak area). The LOD, linear range (LR), enrichment factor (EF) and absorption capacity (AC) were obtained 35 ng L-1, 0.15 -7.2 μg L-1, 19.8 and 131.6 mg g-1, respectively.
References
A. Abbas, A.M. Al-Amer, T. Laoui, M.J. Al-Marri, M.S. Nasser, M. Khraisheh, Heavy metal removal from aqueous solution by advanced carbon nanotubes: critical review of adsorption applications, Sep. Purifi. Technol., 157 (2016) 141-61.
S. Feng, X. Wang, G. Wei, P. Peng, Y. Yang, Z. Cao, Leachates of municipal solid waste incineration bottom ash from Macao: Heavy metal concentrations and genotoxicity, Chemosphere., 67 (2007) 1133-1137.
S.K. Seilkop, A.R. Oller, Respiratory cancer risks associated with low-level nickel exposure: An integrated assessment based on animal, epidemiological, and mechanistic data, Regul. Toxicol. Pharm., 37 (2003) 173–190.
Agency for Toxic Substances and Disease Registry, Division of Toxicology and Human Health Sciences, 1600 Clifton Road NE, Mailstop S102-1, Atlanta, GA 30333, revision 2019.
Agency for Toxic Substances and Disease Registry (ATSDR), Toxicological profile for Nickel. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service, 2005.
N. Altunay, A. Elik, R. Gürkan, Vortex assisted-ionic liquid based dispersive liquid liquid microextraction of low levels of nickel and cobalt in chocolate-based samples and their determination by F-AAS, Microchem. J., 147 (2019) 277-285.
L. A. Escudero, A. J. Blanchet, L. L. Sombra, J. A. Salonia, J. A. Gasquez, Determination of the total and extractable fraction of Ni in lake sediments and natural waters of San Luis (Argentina) by F-AAS using a simple solid phase extraction system, Microchem. J., 116 (2014) 92-97.
M. Felipe-Sotelo, A. Carlosena, J. M. Andrade, M. J. Cal-Prieto, D. Prada, Slurry-based procedures to determine chromium, nickel and vanadium in complex matrices by ET-AAS, Microchem. J., 81 (2005) 217-224
A. Baysal, S. Akman, A rapid solid sampling method for determination of nickel and copper along human hair by ET-AAS, Microchem. J., 98 (2011) 291-296.
G. Özzeybek, B. Alacakoç, Trace determination of nickel in water samples by slotted quartz tube-flame atomic absorption spectrometry after dispersive assisted simultaneous complexation and extraction strategy, Environ. Monit. Assess., 190 (2018) 498.
J. Shan Qun, W. Xiang Yu, S. Jin Lyu, Analysis of nickel distribution by synchrotron radiation X-ray fluorescence in nickel-induced early- and late-phase allergic contact dermatitis in Hartley guinea pigs, Chinese Med. J., 132 (2019)1959-1964.
S. Kanchi, B. Ayyappa , M. Sabela , K. Bisetty, N Venkatasubba Naidu, Polarographic Interaction of Nickel (II) with Ammonium Piperidine-1- Carbodithioate: Application to Environmental Samples, J. Environ. Anal. Chem., 1 (2014) 107.
Y. Min Park, J. Yeon Choi, E. Yeong Nho, C. Mi Lee, Determination of macro and trace elements in canned marine products by inductively coupled plasma optical emission spectrometry (ICP-OES) and ICP-mass spectrometry (ICP-MS), J. Anal. Lett., 52 (2019) 1018-1030.
S. C. Wilschefski, M. R. Baxter, Inductively coupled plasma mass spectrometry: introduction to analytical aspects, Clin. Biochem. Rev., 40 (2019) 115-133.
A. Fadhil Khudhair, M. Khudhair Hassan , H. F. Alesary , A. S. Abbas, Simple pre-concentration method for the determination of nickel(II) in urine samples using UV-VIS spectrophotometry and flame atomic absorption spectrometry techniques, Indones. J. Chem., 19 (2019) 638 – 649.
G. Saravanabhavan, K. Werry, M. Walker, D. Haines, M. Malowany, C. Khoury, Human biomonitoring reference values for metals and trace elements in blood and urine derived from the Canadian Health Measures Survey 2007–2013, Int. J. Hyg. Environ. Health, 220 (2017) 189–200.
S. M. Sorouraddin, M. A. Farajzadeh H. Nasiri, Picoline based-homogeneous liquid–liquid microextraction of cobalt(II) and nickel(II) at trace levels from a high volume of an aqueous sample, Anal. Methods, 11 (2019) 1379-1386.
S.Z. Mohammadi, H. Hamidian, L. Karimzadeh, Z. Moeinadini, Simultaneous extraction of trace amounts of cobalt, nickel and copper ions using magnetic iron oxide nanoparticles without chelating agent, J. Anal. Chem., 68 (2013) 953–958.
A. Hol, A. Akdogan, A.A. Kartal, U. Divrikli, L. Elci, Dispersive liquid-liquid microextraction of nickel prior to its determination by micro sample injection system-flame atomic absorption spectrometry, Anal. Lett., 47 (2014) 2195–2208.
Q. Han, Y. Huo, L. Yang, X. Yang, Y. He, J. Wu, Determination of trace nickel in water samples by graphite furnace atomic absorption spectrometry after mixed micelle-mediated cloud point extraction, Molecul., 23 (2018) 2597.
S. Davari, F. Hosseini, H. Shirkhanloo, Dispersive solid phase microextraction based on amine-functionalized bimodal mesoporous silica nanoparticles for separation and determination of calcium ions in chronic kidney disease, Anal. Methods Environ. Chem., J. 1 (2018) 57-66.
H. Shirkhanloo, Z. Karamzadeh, J. Rakhtshah, N. Motakef Kazemi, A novel biostructure sorbent based on CysSB/MetSB@MWCNTs for separation of nickel and cobalt in biological samples by ultrasound assisted-dispersive ionic liquid-suspension solid phase micro extraction, J. Pharm. Biomed. Anal., 172 (2019) 285-294.
M. Behbahani1, A.Veisi, F. Omidi, A. Noghrehabadi, A. Esrafili, M. Hossein Ebrahimi, Application of a dispersive micro‐solid‐phase extraction method for pre‐concentration and ultra‐trace determination of cadmium ions in water and biological samples, Appl. Organometal. Chem., (2017) e4134.
M. A. Habila, Z. A. ALOthman, E. Yilmaz, M. Soylak, Activated carbon cloth filled pipette tip for solid phase extraction of nickel(II), lead(II), cadmium(II), copper(II) and cobalt(II) as 1,3,4-thiadiazole-2,5-dithiol chelates for ultra-trace detection by FAAS, Int. J. Environ. Anal. Chem., 98 (2018) 171-181.
M.R. Pourjavid, M. Arabieh , A. A. Sehat, Study on column SPE with synthesized graphene oxide and FAAS for determination of trace amount of Co(II) and Ni(II) ions in real samples, Mater. Sci. Eng. C, 47 (2014) 114-122
S. N. Nabavi, S. M. Sajjadi, Z. Lotfi, Novel magnetic nanoparticles (CoO–Fe2O3@SiO2@TiO2) as adsorbent in ultrasound-assisted micro-solid-phase extraction for rapid pre-concentration of some trace heavy metal ions in environmental water samples: desirability function, Chem. Papers, 74 (2019)1143–1159.
W.S.J. Hummers, R.E. Offeman, Preparation of Graphitic Oxide, J. Am. Chem. Soc., 80 (1958) 1339-1339.
M.K. Abbasabadi, A. Rashidi, S. Khodabakhshi, Benzenesulfonic acid-grafted graphene as a new and green nanoadsorbent in hydrogen sulfide removal, J. Nat. Gas Sci. Eng., 28 (2016) 87-94.
M. Khaleghi-Abbasabadi, D. Azarifar, Magnetic Fe3O4-supported sulfonic acid-functionalized graphene oxide (Fe3O4@GO-naphthalene-SO3H): a novel and recyclable nanocatalyst for green one-pot synthesis of 5-oxo-dihydropyrano[3,2-c]chromenes and 2-amino-3-cyano-1,4,5,6-tetrahydropyrano[3,2-c]quinolin-5-ones, Res. Chem. Intermed.. 45 (2019) 2095–2118.
M. Khaleghi Abbasabadi, A. Rashidi, J. Safaei-Ghomi, S. Khodabakhshi, R. Rahighi, J. A new strategy for hydrogen sulfide removal by amido-functionalized reduced graphene oxide as a novel metal-free and highly efficient nanoadsorbent, Sulfur Chem.. 36 (2015) 660-671.
K.S. Novoselov, Z. Jiang, Y. Zhang, S.V. Morozov, H.L. Stormer, U. Zeitler, J.C. Maan, .S. Boebinger, P. Kim, A.K. Geim, Room-temperature quantum hall effect in graphene, Sci., 315 (2007) 1379.
M.Z. Kassaee, E. Motamedi, M. Majdi, Magnetic Fe3O4-graphene oxide/polystyrene: Fabrication and characterization of a promising nanocomposite, Chem. Eng. J., 172 (2011) 540-549.
S. Khodabakhshi, F. Marahel, A. Rashidi, M. Khaleghi Abbasabadi, A Green Synthesis of Substituted Coumarins Using Nano Graphene Oxide as Recyclable Catalyst, J. Chin. Chem. Soc., 62 (2015) 389-392.
D. Azarifar, M. Khaleghi-Abbasabadi, Fe3O4-supported N-pyridin-4-amine-grafted graphene oxide as efficient and magnetically separable novel nanocatalyst for green synthesis of 4H-chromenes and dihydropyrano[2,3-c]pyrazole derivatives in water, Chem. Intermed., 45 (2019) 199–222.
C. Nethravathi, M. Rajamathi, Chemically modified graphene sheets produced by the solvothermal reduction of colloidal dispersions of graphite oxide, Carbon, 46 (2008) 1994-1998.
D. Chen, H. Zhang, K. Yang, H. Wang, Functionalization of 4-aminothiophenol and 3-aminopropyltriethoxysilane with graphene oxide for potential dye and copper removal, J. Hazard. Mater., 310 (2016) 179-187.
J.S. Wang, R.T. Peng, J.H. Yang, Y.C. Liu, X.J. Hu, Preparation of ethylenediamine-modified magnetic chitosan complex for adsorption of uranyl ions Carbohydr. Polym., 84 (2011) 1169-1175.
X.J. Hu, Y.G. Liu, H. Wang, A.W. Chen, G.M. Zeng, S.M. Liu, Removal of Cu(II) ions from aqueous solution using sulfonated magnetic graphene oxide composite, Sep. Purif. Technol. 108 (2013) 189-195.
L.Z. Bai, D.L. Zhao, Y. Xu, J.M. Zhang, Y.L. Gao, L.Y. Zhao, J.T. Tang, Inductive heating property of graphene oxide–Fe3O4 nanoparticles hybrid in an AC magnetic field for localized hyperthermia, Mater. Lett., 68 (2012) 399-401.
M. Khaleghi Abbasabadi, A. Rashidi, J. Safaei-Ghomi, S. Khodabakhshi, R. Rahighi, A new strategy for hydrogen sulfide removal by amido-functionalized reduced graphene oxide as a novel metal-free and highly efficient nanoadsorbent, J. Sulfur Chem., 36 (2015) 660-671.
S. Khodabakhshi, B. Karami, Graphene oxide nanosheets as metal-free catalysts in the three-component reactions based on aryl glyoxals to generate novel pyranocoumarins, New J. Chem., 38 (2014) 3586-3590.
H. Kim, K.Y. Park, J. Hong, K. Kang, All-graphene-battery: bridging the gap between supercapacitors and lithium ion batteries, Sci. Rep., 4 (2014) 5278.
C. Hou, Q. Zhang, M. Zhu, Y. Li, H. Wang, One-step synthesis of magnetically-functionalized reduced graphite sheets and their use in hydrogels, Carbon, 49 (2011) 47-53.
E. Rafiee, M. Khodayari, Two new magnetic nanocomposites of graphene and 12-tungestophosphoric acid: characterization and comparison of the catalytic properties in the green synthesis of 1,8-dioxo-octahydroxanthenes, RSC Adv., 16 (2016) 36433-36440.
S. Khodabakhshi, M. Khaleghi Abbasabadi, S. Heydarianc, S Gharehzadeh Shirazi, F Marahel, Fe3O4 Nanoparticles as Highly Efficient and Recyclable Catalyst for the Synthesis of 4-Hydroxy-3-[aryloyl(benzamido)methyl]coumarin under Solvent-Free Conditions, Lett. Organ. Chem., 12 (2015) 465-470.
J. Wang, Z. D. Han, The combustion behavior of polyacrylate ester/graphite oxide composites, Polym, Adv. Technol., 17 (2006) 335–340
P. J. Yen and C. C. Ting, et al., Facile production of graphene nanosheets comprising nitrogen-doping through in situ cathodic plasma formation during electrochemical exfoliationJ. Mater. Chem. C, 5 (2017) 2597-2602
C. C. Yang and M. H. Tsai, et al., Carbon Nanotube/Nitrogen-Doped Reduced Graphene Oxide Nanocomposites and Their Application in Supercapacitors, J. Nanosci. Nanotechnol., 17 (2017) 5366–5373.
L. J. Li and C. W. Chu, et al., Plasma-assisted electrochemical exfoliation of graphite for rapid production of graphene sheets, RSC Adv., 4(2014)6946–6949.
M. Rohaniyan, A. Davoodnia, A. Nakhaei, Another application of (NH4)42[MoVI72MoV60O372(CH3COO)30(H2O)72] as a highly efficient recyclable catalyst for the synthesis of dihydropyrano[3,2]chromenes, Appl. Organometal. Chem., 30 (2016) 626-629
J. Wang and Z. D. Han, The combustion behavior of polyacrylate ester graphite oxide composites, Polym. Adv. Technol., 17(2006) 335–340.
X. Wang and L. Zhang, Green and facile production of high-quality graphene from graphite by the combination of hydroxyl radicals and electrical exfoliation in different electrolyte systems, RSC Adv., 9 (2019) 3693-3703.
ZH Mousavi, A Rouhollahi, Preconcentration and determination of heavy metals in water, sediment and biological samples, J. Serb. Chem. Soc., 76 (2011) 1583-1595
HZ Mousavi, A Asghari, Determination of Hg in water and wastewater samples by CV-AAS following on-line preconcentration with silver trap, J. Anal. Chem., 65 (2010) 935-939.
A Khaligh, F Golbabaei, Z Sadeghi, A Vahid, A Rashidi, On-line micro column preconcentration system based on amino bimodal mesoporous silica nanoparticles as a novel adsorbent for removal and speciation of chromium (III, VI) inenvironment samples, J. Environ.Health Sci. Eng., 13 (2015) 47.
Copyright (c) 2020 Analytical Methods in Environmental Chemistry Journal

This work is licensed under a Creative Commons Attribution 4.0 International License.
JOURNAL PUBLISHING AGREEMENT
PLEASE PROVIDE US THE FOLLOWING INFORMATION,
Article entitled:
Corresponding author:
To be published in the journal:
Your Status
I am the sole author of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
I am one author signing on behalf of all co-authors of the manuscript
- I am an Iranian government employee.
- I am a European government employee
- I am a Asian government
- None of the above
Please tick the above blanks (as appropriate), review the Journal Publishing Agreement, and then sign and date the document in black ink.
Published Journal Article: the author may share a link to the formal publication through the relevant DOI. Additionally theses and dissertations which contain embedded Published Journal Articles as part of the formal submission may be hosted publicly by the awarding institution with a link to the formal publication through the relevant DOI. Any other sharing of Published Journal Articles is by agreement with the publisher only.
Signed: ______________________________________ Name printed: ___________________________________________
Title and Company (if employer representative): _______________________Date: __________________________________